Zoe

Photo by Kathy Thiessen
Kathy Thiessen's 10-year-old shih tzu, Zoe, has been taking Vetmedin for a heart condition since March, but the drug is increasingly tough to come by.
Kathy Thiessen vaguely recalls a veterinarian telling her of Zoe's heart murmur in 2018. She didn't think much of it at the time, assuming her then-8-year-old shih tzu had a benign condition.
But in March, Zoe cracked a tooth while chewing a bone and needed to have it pulled. The procedure, which required Zoe to be anesthetized, called for a full examination, and the murmur again was detected. X-rays revealed an enlarged heart.
Zoe's veterinarian prescribed Vetmedin (pimobendan), a life-extending cardiac medication for dogs with heart disease. "She told me that with this medication, it would give Zoe a couple more years than without it," said Thiessen, of Everett, Washington. "I've owned pets my whole life, and my husband and I both agree that this dog is our favorite. If there's a medication that can extend her life, there's no way we won't get it for her."
That is, if it's available.
Vetmedin has gone through periods of scarcity since April 2007, when the U.S. Food and Drug Administration approved it for use in dogs. New challenges again have the medication in short supply, much like it was in September 2019, when a regulatory paperwork issue caused production to temporarily cease at the Mexico City facility where Vetmedin is manufactured. Supplies normalized within a couple of months.
This time around, supply interruptions are expected to persist through the end of the year, according to Randolph Legg, head of U.S. Boehringer Ingelheim Animal Health, maker of Vetmedin. In a "Dear Doctor" letter dated Aug. 25, he wrote that the company is "committed to re-establishing a robust supply as quickly as possible" and "urgently pursuing a number of actions" to normalize production.
The COVID-19 pandemic's impact on Mexico City, where Vetmedin continues to be manufactured, is among "multiple factors" causing the latest slowdown, said Heidi De Wit-Sommerfeld, head of U.S. communications for Boehringer Ingelheim Animal Health, maker of Vetmedin. "While we are pursuing a number of actions, Vetmedin is being allocated to veterinarian customers across all presentations to ensure the best possible availability of product for pet patients," she said by email.
De Wit-Sommerfeld didn't explain what the allocation process looks like, but some veterinary and retail pharmacies are short on Vetmedin, and if they have stock to sell, it doesn't come in all dosages. In the U.S., Vetmedin typically comes in 1.25 mg, 2.5 mg, 5 mg and 10 mg chewable tablets.
After calling around, Thiessen said she recently "got lucky" and found a two-month supply of 1.25 mg Vetmedin from a nearby Costco pharmacy for $170. Zoe is prescribed to take one 2.5 mg tablet in the morning and a half-dose at night. Thiessen makes do by giving her dog three 1.25 mg tablets a day.
"My dog's life is dependent on this," she said. "I should have bought more when I could."
No substitute for pimobendan
Vetmedin bottle
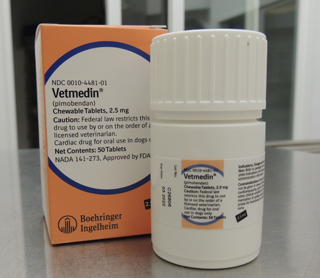
Photo by Caitlin Denney
Vetmedin (pimobendan) is in short supply. The drug is used to treat dogs with dilated cardiomyopathy or valvular insufficiency, both prior to and after developing congestive heart failure, as well as subclinical mitral valve disease.
Pimobendan, the active ingredient in Vetmedin, is the first drug in a class of heart medications called inodilators approved for oral administration in dogs. It does two things to help the heart pump more efficiently: It opens blood vessels that take in blood from the heart, lessening its workload, and increases the strength of heart-muscle contractions.
The drug often is prescribed to treat heart failure due to mitral regurgitation and dilated cardiomyopathy. In rare cases, it's prescribed off-label for feline heart conditions.
While the FDA Center for Veterinary Medicine acknowledges that pimobendan is important to animal health, it is not on the agency's list of medically necessary drugs that regulators track to help alleviate or prevent shortages.
However, if supply problems persist long enough, the agency can get involved. "CVM continues to monitor the situation with pimobendan," FDA spokesperson Juli Putnam said by email. "CVM has reached out to the sponsor [manufacturer] again for information about the current situation related to this product to allow us to determine the status."
Pimobendan preparations can be made-to-order by several compounding pharmacies; however, their efficacy has not been studied to any great extent. And no substitutes of the drug exist, said Dr. Mark Kittleson, a veterinary cardiologist and professor emeritus of the University of California, Davis, School of Veterinary Medicine.
While drugs such as dobutamine and dopamine are potent positive inotropes — agents that increase the strength of muscular contraction — they can only be given for a short time intravenously. Digoxin, a weak positive inotrope, also cannot be used as a substitute.
The latest pimobendan shortage, combined with lacking alternatives prompted a recent online petition, asking BI Chief Executive Officer Dr. Wolfgang Baiker to "do absolutely everything" to resolve the issue, "whether it be having your company send out surveys to vet hospitals, reviewing the annual number of dogs needing this medicine and/or having a survey sent out to distributors on how many orders they receive per year." To date, the petition has 2,367 signatures.
Practitioners on Veterinary Information Network, an online community for the profession and parent of the VIN News Service, also have expressed concerns about Vetmedin supply disruptions.
Dr. Mary Kinser, a practitioner in Greenville, Michigan, said by email that she had no issue getting Vetmedin in her practice until a few weeks ago, when suddenly, her orders stopped coming. Trying to make do with stock she had on hand, she asked colleagues in a VIN message board post about whether it's safe to split larger milligram tablets to achieve an intended lower dosage.
Kittleson, a VIN consultant, responded. "I see no real problem with quartering the tabs as long as they break relatively cleanly," he said.
Update: Vetmedin remains in short supply as of February 2021, according to Boehringer Ingelheim Animal Health. "The production site [in Mexico] has unfortunately been severely affected by COVID-19, so additional measures were implemented to ensure social distancing in lab and production areas, demanding re-organization of teams and further process optimization," reports Heidi De Wit-Sommerfeld, head of U.S. communications. She expects the issue to be resolved with the next few months.
Update 5/12/2021: In response to the continued Vetmedin shortage, the U.S. Food and Drug Administration announced that the agency will not block the importation of Vetmedin capsules and chews from Canada, the United Kingdom and Ireland to immediately increase U.S. supplies. There is no FDA-approved alternative to Vetmedin. Vetmedin capsules, chews and chewable tablets all contain the same active ingredient, pimobendan.