New federal rules take effect Oct. 9
VIN News Service photo
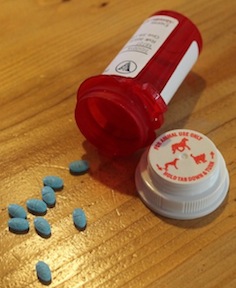
Users of pharmaceutical controlled substances, such as this anti-anxiety medication prescribed to a dog to calm its fear of fireworks, have few safe and legal avenues for disposing of surplus or expired product. Federal rules that take effect Oct. 9 aim to broaden disposal options for patients and (in the case of animal patients) their owners.
Users of controlled medications, including the owners of pets prescribed such drugs, may see more options for disposing of leftover or expired product under new federal rules that take effect next month.
Prompted by the Secure and Responsible Drug Disposal Act of 2010, the regulations open the way for businesses, including retail pharmacies and clinics with on-site pharmacies, to collect surplus controlled drugs for disposal.
Until now, the only authorized disposal options for users of controlled drugs was to turn the product over to law enforcement, avail themselves of periodic “take-back” events, or flush the substances down a sink or toilet.
As a result, drugs accumulated in consumers’ medicine cabinets, susceptible to diversion — falling into the wrong hands for sale or abuse — or of being ingested by accident. Flushing unused medications, once standard advice, has been found to pollute waterways.
“Prescription drug abuse is an American epidemic, and it is America’s fastest growing drug problem,” the U.S. Department of Justice Drug Enforcement Agency (DEA) states in a Sept. 9 Federal Register notice spelling out the new rule and its rationale. “When large volumes of pharmaceutical controlled substances accumulate, they become an attractive target for drug seekers and drug abusers.”
Controlled drugs are those with the potential to be abused and/or cause dependence. The government classifies them in five categories, or schedules, based upon the likelihood of abuse or dependence and their currently accepted medical use. The new disposal rules apply to schedule II through V drugs. Examples of common controlled pharmaceuticals are OxyContin, Ritalin, Tylenol with codeine, ketamine, Xanax, Valium and Ambien.
Schedule I drugs, along the lines of heroin and marijuana, have no recognized medical use in the view of the federal government and are illegal under U.S. law, so they aren’t allowed at authorized collection sites.
The rule change, effective Oct. 9, allows a variety of parties that are registered to handle controlled pharmaceuticals, from distributors to doctors, to collect unwanted controlled drugs as a service to customers and clients. They may set up secure collection bins at their respective registered locations or, if they are able to destroy the discards on-site, they may accept the drugs by mail in tamper-proof packaging.
Disposal providers must be registered with the DEA and have the authority to possess and dispense schedule II drugs. Registrants may apply online at no charge to become a controlled-substances collector. The role is voluntary, not required of registrants.
How many veterinary clinics would opt to become collectors is an open question. They would have to fund the service themselves, such as by paying a company known as a “reverse distributor” to retrieve the contents of the collection bin for destruction.
“It would add burden and liability to the practitioners, but if they see it adds benefit to the clinic as a service to clients, and they qualify (under DEA rules), it could be a way for them to stand out,” said Dr. Kristi Henderson, assistant director of the American Veterinary Medical Association (AVMA) scientific activities division.
The DEA suggests in the Federal Register notice that collectors may benefit from “increased consumer presence at their registered locations and the goodwill that develops from providing a valuable community service.”
One way practitioners might participate without hosting a collection bin would be to distribute mail-back envelopes that are addressed to a business capable of destroying the unused drugs through a method such as incineration, according to the DEA.
In addition to trying to make it easier for consumers to safely shed unused controlled medications, the revamped federal rule makes modest changes to the disposal procedures that DEA registrants must follow.
Unlike patients or owners of animals prescribed controlled substances, dubbed by the DEA as “ultimate users,” doctors who are authorized to possess and dispense controlled drugs aren’t allowed to dispose of excess or expired products through collection bins or take-back programs.
How clinicians may dispose of surplus inventory legally and affordably is a perplexing question that arises periodically on message-board discussions on the Veterinary Information Network, an online community for the profession.
Dr. Gary Lainer, a veterinarian in Massachusetts, commented in July: “In the past few months, we have accumulated vials and patches of outdated schedule drugs. … We keep well-documented drug-usage books, but I don't see any forms or protocols for expired controlled substances. Is this is a state or federal domain? … No one seems to know what is required.”
In an interview by email, Lainer expressed frustration about the lack of clear instructions for lawful disposal by practices.
“I have called my local police department, my state Division of Licensing and Drug control, my pharmaceutical distributor (figuring they gave me the drugs, so they should take the expired ones back), and each has explained that this is not their domain,” he said.
“There are companies willing to take these drugs for a significant fee, and I'm considering going this route,” Lainer continued. “However, there should be a comprehensive program for any expired controlled substance in order for practitioners to correctly comply with the law. At the present time, the DEA and their state divisions have not spelled this out very well.”
He added: “I'm absolutely sure there are many out there just spilling the liquids into the drain, crushing pills and throwing them in the trash and unknowingly breaking the law. I've been trying to do the right thing, but honestly, I'm not 100 percent sure of what I should be doing.”
The DEA has had a procedure for registrants to dispose of unwanted controlled drugs, but the old rules were scattered in different sections of the Code of Federal Regulations. Those regulations basically required practitioners to consult with the DEA Special Agent in Charge in their region. They needed to fill out paperwork (Form 41) and submit three copies to the agent. The agent would specify what to do.
The revised regulation consolidates the directions in one section (Part 1317) and provides some options that don’t require direction from the Special Agent in Charge, although they still require record-keeping.
One option is to directly destroy the substance, provided the registrant has at his or her registered site a method of destruction that is legal under local, tribal, state and federal law. The method must render the substance “non-retrievable,” which is defined as “permanently alter[ing] the substance’s physical or chemical condition or state through irreversible means [so as to make it] unavailable and unusable for all practical purposes.”
The DEA notes that “The process to achieve a non-retrievable condition or state may be unique to a substance’s chemical or physical properties. A controlled substance may be considered ‘non-retrievable’ when it cannot be transformed to a physical or chemical condition or state as a controlled substance or controlled-substance analog.”
If a registrant opts to destroy the substance on-site, the job may not be done solo. The regulations state that "Two employees of the registrant must handle or observe the handling of any controlled substance until the substance is rendered non-retrievable."
The agency states elsewhere in the 52-page Federal Register notice that mixing controlled substances with kitty litter or coffee grounds and disposing of the amalgam in the garbage does not meet the “non-retrievable” standard.
The agency opted not to name specific approved methods of disposal because it “hopes that the rule will encourage innovation and expansion of destruction methods beyond incineration …”
However, the DEA says it “will not be routinely engaged in evaluating new technologies intended to render controlled substances 'non-retrievable.' "
Moreover, the DEA suggests that choosing a disposal method is up to the judgment of the registrant. It writes, “… the DEA will not evaluate, review or approve the processes or methods utilized to render a controlled substance non-retrievable, as long as the desired result is achieved."
Another option for registrants is to send unwanted product to a reverse distributor, a company that charges a fee for the service. In the case of returns or recalls, they may send it to the individual, manufacturer or representative of the manufacturer from whom it was obtained.
Registrants with product to dispose of also still have the option of consulting the Special Agent in Charge. If consulting the agent, they will need provide only one copy, rather than three, of Form 41.
A recent blog post by the AVMA’s Henderson outlines what the new rule means for veterinary practices.
The revisions represent a somewhat more efficient process for registered practitioners, Henderson said in an interview, although, she noted, the thrust of the new rule is easing disposal by consumers to safeguard children, pets and the environment and to avert diversion. “That’s why we’re excited about it,” she said. “It helps practitioners a little, (and) it helps the general public a lot.”