Long-anticipated policy gives broad latitude in prescribing for individual patients
Compounding illo

Guidance from regulators on a notoriously perplexing subject — veterinary drug compounding — is, at long last, completed.
Producing the guidelines took the U.S. Food and Drug Administration nearly 20 years, illustrating the difficulty of the endeavor. The agency's task was to specify the conditions under which it would let veterinary medicines be made through a process that technically is illegal.
Compounding is a practice by which drugs are altered in dosage, form and/or flavor to accommodate the needs of a particular patient. They are created outside of the FDA drug-approval process and, as such, are not vetted for safety and efficacy.
The agency's challenge was to enable veterinarians to meet the widely varying needs of their animal patients without providing a loophole through which businesses could sidestep the government's rigorous drug-approval process.
The result, Guidance For Industry #256 - Compounding Animal Drugs from Bulk Drug Substances, released on April 13, seems to have achieved the desired balance, judging from early positive reviews from veterinary pharmacy experts.
"Overall, I think it is well thought out and addresses a lot of the concerns that will arise," said Lauren Forsythe, medication dispensary coordinator at the University of Illinois at Urbana-Champaign Veterinary Teaching Hospital.
Dr. Dawn Boothe, a veterinarian and director of the Clinical Pharmacology Laboratory at Auburn University College of Veterinary Medicine, agreed. "This has been a tough, tough document to produce for so many reasons. I applaud them," she said.
"Their document demonstrates that they have listened to the constituents that weighed in, and they thought long and hard about trying to meet the needs of all the practitioners," Boothe said, "and I think they've given us the tools to do this as safely and as appropriately and as ethically as possible."
A blog post by the American Veterinary Medical Association notes, "Throughout the FDA's development of this guidance ... the AVMA has actively communicated our profession's needs for compounded products. ... The final guidance reflects many changes made in response to advocacy by the AVMA."
Because veterinary patients come in so many shapes and sizes, including many species for which no drugs are made at all, compounding is an essential tool in veterinary medicine. The law allows FDA-approved drugs to be manipulated for compounded preparations but it does not allow compounded drugs to be made from raw materials, known as "active pharmaceutical ingredients" or "bulk drug substances."
Compounding pharmacists say it is impossible to use only approved finished drugs as the base for custom preparations, and the FDA acknowledges the point. In the guidance, the agency lays out conditions under which it will opt not to enforce the law, thereby de facto allowing bulk substances to be used. The guidance is not a regulation but is meant to provide a window to the FDA's thinking.
The particulars
Broadly, the FDA approaches the issue in two ways, depending on the circumstance.
For individual patients that are not animals used for food, veterinarians may compound drugs or prescribe compounded drugs made from any active pharmaceutical ingredient, as long as the product is not a copy of an approved or indexed drug on the market. (Indexed drugs are those made for certain animal species under an alternative review process.)
The prohibition on drug copies is not absolute. Copies may be compounded if there is a medical rationale that the compounded version makes a clinical difference in the patient. An example of a medical rationale is that a patient rejects an approved drug because of its flavor but accepts a compounded version made with different flavoring. Other examples are that the patient is allergic to an ingredient in an approved drug, or the patient would require a fraction of an approved tablet and the tablet isn't scored for the needed fraction. The prescriber must give the medical rationale to the pharmacist, and the pharmacist must keep a record of it.
Explanations that the FDA does not consider medical rationales are that a compounded preparation costs less, or that the prescriber or pet owner simply prefers to use a compounded drug or compounding pharmacy.
Beyond compounding for individual pets, the FDA takes a much more hands-on approach. Compounders who produce office stock — medications that veterinarians keep on hand to administer to any number of unspecified patients — should not use bulk drug substances except in limited instances. The instances are spelled out on an FDA-created List of Bulk Drug Substances for Compounding Office Stock Drugs for Use in Nonfood-Producing Animals.
The list is dynamic. Anyone may nominate a drug for the list, and the FDA will review the request. The agency won't take enforcement action against a compounder using bulk drug substances that are being considered for inclusion on the list.
For animals used as food and for free-ranging wildlife, there is a separate List of Bulk Drug Substances for Compounding Drugs for Use in Food-Producing Animals or Free-Ranging Wildlife Species.
The reason that drugs compounded for office stock are treated differently from drugs made for an individual is that office stock will reach many more patients, said Dr. Amber McCoig, an FDA senior veterinary medical officer and member of the agency's veterinary compounding team.
"If there is any issue with the quality, or anything that would potentially harm the animal, it would affect more animals altogether," McCoig said in an interview. "It's also held in the office for an undetermined amount of time, and we don't know about the stability of the product."
One aspect of the guidance that affects all compounded preparations, whether for individuals or office stock, is labeling. The guidance calls for the labeling to have the following:
- the drug name
- the drug strength
- species of the patient
- patient identification, such as its name or a descriptor like "horse in stall X" or "dogs in shelter kennel X" (not needed for office stock)
- name, address and contact information for the compounding pharmacy or compounding veterinarian, and name of veterinarian prescribing the medication or ordering the office stock
- beyond-use date
- the statement "Report suspected adverse reactions to [pharmacist or veterinarian who compounded the drug] and to FDA online using Form FDA 1932a"
- the statement "This is a compounded drug. Not an FDA approved or indexed drug"
- the statement "Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian"
The advice to tell the FDA about adverse events on compounded drugs is new. In the past, the agency asked that such reports be made only to the compounders, McCoig said. Now, "we're asking that they be reported to the compounders and the FDA [because] we don't know all the adverse events that may be happening."
An adverse event is any undesirable experience associated with the use of a medical product in a patient, according to the FDA. In drugs, this includes serious side effects, problems with product use or quality, and therapeutic failure — meaning that a drug doesn't have its intended effect.
Boothe, the veterinary clinical pharmacologist at Auburn University, said she is "tickled pink" by the attention to adverse-event reporting. "Our profession in general does not do a good job in reporting adverse events," she said. "We get too complacent and too cavalier, and yet it is one of the best ways for us to ensure safety of drugs."
Questions remain
Vet-Compounding-Guidance
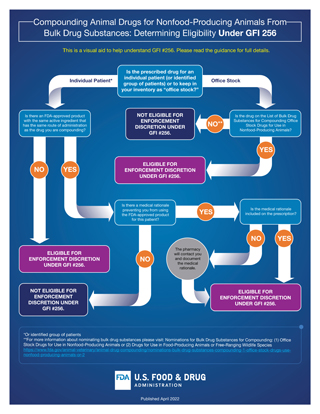
Image courtesy of FDA
This flow chart is one of several resources the U.S. Food and Drug Administation has created to help veterinarians learn about the agency's new guidance on compounding using bulk drug substances. Others are a
Q&A and a
checklist for veterinarians.
Click here for a larger view
The 22-page guidance leaves some questions unanswered. For example, it doesn't say whether a shortage of an approved or indexed drug constitutes an acceptable rationale for prescribing a compounded copy. McCoig said decisions on drugs affected by shortages will be made case-by-case.
The guidance doesn't address anticipatory compounding, such as a pharmacy making a preparation in advance of a veterinarian's expected prescription. McCoig said as long as the prescription is made for a specific patient or group of patients, anticipatory compounding would be considered in the category of patient-specific prescriptions, not office stock.
The guidance also doesn't mention "503B outsourcing facilities," a classification of compounding pharmacy that was created under the Drug Quality and Security Act (DQSA) of 2013 to regulate drug compounding for human patients. There are about 75 registered 503B outsourcing facilities, some of which sell compounded drugs for veterinary as well as human use.
McCoig said the FDA does not track how many of them compound drugs for veterinary use. In any case, she said that the agency would apply "enforcement discretion" with those facilities compounding animal drugs with active pharmaceutical ingredients, provided they follow the guidance.
Marcy Bliss, CEO of Wedgewood Pharmacy, one of the largest veterinary compounders in the country and operator of a 503B facility called Wedgewood Connect, said the impact of the guidance on large compounding businesses like hers is uncertain.
One possibility is that prices on compounded medications will rise, she said. "It costs more for a pharmacy to [compound medications] on a patient-specific basis," Bliss explained, "because you're gathering more information: name, species ... You have to have a pharmacist take that information and then you have to label every product with that information. ... It makes it more expensive to do it that way, and I presume that more pharmacies will have to pass those expenses on to the pet owner."
Briefly describing her company's operation, Bliss said Wedgewood uses 450 active pharmaceutical ingredients to create 45,000 discrete products. "If there's something that's used by a lot of veterinarians, we may have it in seven dosage forms and, like, 140 strengths," she said, not to mention multiple flavors.
Bliss said Wedgewood believes that "veterinarians should decide what's appropriate based on their clinical experience and their clinical observations, and this [regulatory agency action] gets in the middle of that. ... Why do they have to prove or document their clinical rationale?"
Asked whether Wedgewood would legally challenge the FDA guidance, she said, "We are still evaluating that. We have a call next Tuesday with a coalition of pharmacies that specialize in animal health, and we want to gather their thoughts on whether this is workable for their patients and their veterinarians or not, and we're gathering our thoughts, as well."
Long, winding history
Gigi Davidson, a pharmacist who has been engaged in the veterinary compounding policy arena for decades, including as a member of an FDA group that created the agency's first guidance on the subject in 1993, praised the agency's meticulous legal documentation in the new final guidance.
"There are twice as many footnotes and statutory references as in the draft [that was issued in 2019] — 37 in the final versus 20 in the draft," she observed. "So if you go through, you can see that they have been very, very careful to reference every one of their statements in the rule of law when the rule of law is available. That, in my opinion, has resulted in an overall tone for the finalized guidance that is less editorial and more authoritative."
Its creation illustrates the saying, "If at first you don't succeed, try, try again." The odyssey dates to 2003, when the FDA issued a draft update to its original 1990s-era guidance. The update made no allowances for the use of bulk drug substances for companion animals. The resulting furor led the agency in 2004 to announce it would revise the draft and make a new version available for comment later in the year. That didn't happen.
Years went by. Not until 2015 did the agency offer a new draft. But in 2017, it backed away from that approach, too.
In 2019, the FDA offered yet another rendition. It twice extended the public comment period, in part because of the Covid-19 pandemic. Then, this month, following changes to the draft, the long-awaited guidance was made final.
Understanding that the subject and the agency policy aren't simple, FDA officials said the agency will spend coming months doing outreach and education in the veterinary community to answer questions about the guidance and its implementation. The FDA will not shift its focus to pharmacy inspections related to the new policy until October or later, and it does not intend to inspect veterinary clinics, McCoig said.