FPV medication
Photos courtesy of Dr. Elizabeth Coney
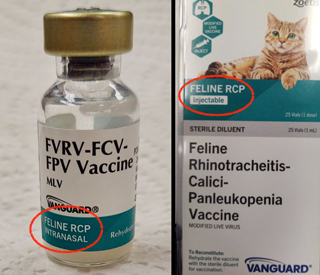
Zoetis mistakenly labeled vials of its injectable feline RCP vaccine "intranasal," which contradicts outer packaging that accurately identifies the product as "injectable."
Zoetis has recalled an untold amount of Vanguard Feline RCP due to a packaging error that mislabeled vials of the injectable vaccine by listing "intranasal" as the route of administration.
"This product is only approved for subcutaneous or intramuscular injectable administration in felines," company officials confirmed in a statement to the VIN News Service. The vaccine is intended to protect cats against feline herpesvirus-1, feline calicivirus and feline parvovirus.
Zoetis is asking all veterinarians who have received mislabeled product to return it to the company. Officials will start contacting distributors and veterinary practices next week with steps for returning product and receiving replacements, "rounded up to the next full tray," the statement reads. Customers with immediate questions can call Zoetis at (888) 963-8471.
As of Thursday afternoon, the recall had not been announced on the company's website.
Veterinarians say it's a big deal if cats inhale vaccines that are meant to be injected.
Dr. Elizabeth Coney of Lexington, Kentucky, reported the label discrepancy on Tuesday in a message board post on the Veterinary Information Network, an online community for the profession and parent of the VIN News Service. She said that the outer package containing a tray of Vanguard Feline RCP lists the vaccines as "injectable," while the individual vials are labeled "intranasal."
"This is dangerous," she wrote. "Already had a fellow DVM wonder if this mislabeled product could be given either SQ [subcutaneous] or IN [intranasal]."
True intranasal vaccines contain viruses that are specially attenuated to grow only at cooler temperatures on the surface of the membranes of the nose, said Dr. Alice Wolf, a board-certified internist and VIN consultant. According to information posted by the University of California, Davis, Koret Shelter Medicine Program, "Modified live parenteral feline respiratory virus vaccines are temperature sensitive mutants that depend on being given at the higher core body temperature in order to reduce virulence."
If cats are given an injectable vaccine in their nose, it would not be unusual for them to exhibit signs of severe upper respiratory disease, Wolf said. "It may even cause systemic signs of fever, coughing and anorexia."
Cats are so sensitive, she said, that if injectable vaccine lands on their fur and they lick it off, ulcers can develop in their mouths.
The U.S. Department of Agriculture Animal and Plant Health Inspection Service, which regulates veterinary vaccines, told VIN News today that it's aware of the recall.
"Veterinarians who have received mislabeled vaccine will be hearing directly from the company very soon, if they have not already been alerted," APHIS Public Affairs Specialist Marquita Bady said by email.