 Quantities of key antimicrobial drugs sold for use in food animals rose in the United States in 2019 but authorities maintain that efforts to curb their overuse are still working.
Quantities of key antimicrobial drugs sold for use in food animals rose in the United States in 2019 but authorities maintain that efforts to curb their overuse are still working.
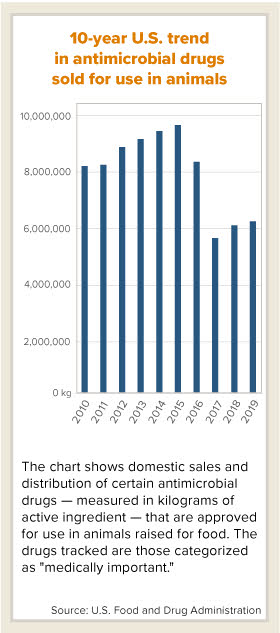
Sales for production animals of "medically important" antimicrobials, which are drugs also used in human medicine, rose 3% to 6,189,260 kilograms of active ingredient, the U.S. Food and Drug Administration reported this week. The FDA added that "the trend over time" remains encouraging. Sales of medically important antimicrobials, the agency noted, were still down 25% since 2010 and 36% since 2015, which was the peak year for sales.
Overuse and misuse of antimicrobial drugs can contribute to the evolution of pathogens that tolerate treatments meant to kill them, endangering the health of humans and other animals.
Fluctuations in sales volumes can be influenced by various factors, including changes in animal populations or health needs. Overuse has been discouraged in many countries, including in the U.S., United Kingdom and individual EU member states, via rule changes and public awareness campaigns.
The FDA had anticipated a partial rebound in sales to continue in 2019, following a steep drop in 2017 caused by a watermark rule change. Sales of medically important antimicrobials rose in 2018, too — by 9% compared with 2017.
In 2017, the FDA banned the use of antimicrobials for promoting growth in livestock, a quirk of the drugs that led some food producers to use them for non-medical reasons. The rules now prohibit over-the-counter use of such drugs in animal feed and water, and require oversight by veterinarians. A bounce back in sales "in subsequent years" after 2017 was not unexpected as affected stakeholders adjusted to the new requirements, the FDA said this week.
More broadly in the U.S., domestic sales of antimicrobials for production animals including those that are "non medically important," such as ionophores, fell by less than 1% compared with 2018, to 11,468,357 kilograms of active ingredient, according to the FDA's 2019 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals.
British usage also back on the rise
Like the U.S., the United Kingdom also recently released figures showing only a moderate change in demand last year, ending a recent multi-year downturn, as further big drops become harder to achieve.
Sales of veterinary antibiotics there rose 3% in 2019 compared with 2018, but were still down 43% compared with 2015, according to a report released on Nov. 18 by the Veterinary Medicines Directorate.
Encouragingly, sales of drugs classified as "highest-priority critically important antibiotics," or HP-CIAs, in food-producing animals in the U.K. dropped 22% in 2019, bringing the total decline in that category to 72% since 2015. Sales of HP-CIAs, however, represent but a small proportion (0.5%) of overall antibiotic sales for animal species, the VMD said.
Strictly defined, antibiotics are a subset of antimicrobial drugs but the terms often are used interchangeably. HP-CIAs are selected by the World Health Organization as among the most important antibiotics for human health. According to the latest version of its list, published in 2018, they are quinolones, third- and higher-generation cephalosporins, macrolides and ketolides, glycopeptides and polymyxins.
VMD chief executive Peter Borriello noted that even though overall antibiotic use had risen in the U.K., sales in 2019 still represented the second-lowest level since counting began, second only to 2018.
"It is pleasing to see that use of HP-CIAs remains very low and continues to fall, helping to protect their effectiveness in human health (and in animal health, if no other antibiotic will work)," Borriello wrote in a foreword to the full report. "This move to less potent antibiotics may in part explain the small increase in total antibiotic use."
Separately, the European Medicines Agency (EMA) recently released figures showing antimicrobial use for animals continuing to fall in the European Union, though its latest numbers were for 2018. The EMA reported on Oct. 21 that in 25 European countries that provided veterinary antimicrobial sales data for all years between 2011 and 2018, there was an overall sales decline of 34.6%.
The EMA reports sales of antimicrobial drugs in milligrams of active ingredient per "population correction unit," or PCU, a proxy that accounts for the size of the food animal population. The amount sold in 2018 from 31 European countries was 103.2 mg per PCU, down 5.6% from the 109.3 mg reported for 2017.
The European Union banned the use of antimicrobial drugs for growth promotion more than a decade earlier than the U.S., in 2006.