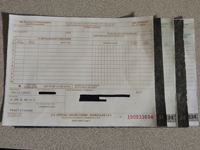
DEA Form 222 320
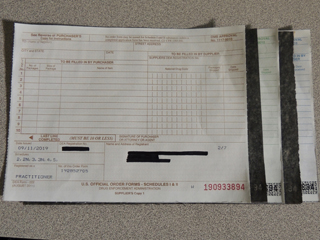
Photo courtesy of Poland Veterinary Centre
The carbon-paper triplicate DEA Form 222, which dates back more than 40 years, is being retired in favor of a form that may be copied digitally or with a photocopier.
The carbon-papered triplicate version of DEA Form 222 is on the way out.
Tomorrow, the U.S. Drug Enforcement Agency rolls out a modern take on the paperwork that health-care providers must use when ordering Schedule I and Schedule II controlled drugs.
The new format consists of a single paper sheet. Rather than manually creating carbon copies, users will fill out the single sheet and make a paper or electronic copy for their records, such as by using a photocopier or scanner, before sending the original to their supplier.
The old three-part form, described by the DEA as "printed on interleaved carbon sheets," was created more than 40 years ago, according to the agency. "[P]rocessing a transaction with carbon copies is outdated," the agency deadpanned in a Federal Register notice published earlier this year about the plan.
"Today," the notice went on, "new office technology exists, such as laser printers, scanners and photocopiers, which will allow DEA registrants greater ease in utilizing the single-sheet form."
Not only that, the DEA said it stands to save hundreds of thousands of dollars a year by updating the form. "There is only one vendor that produces the current three-part carbon forms, which is costly," the notice explains. "The Dot Matrix printer used to print the forms is outdated, and DEA can only get replacement parts from one vendor. Maintaining the equipment is costly, difficult and time-consuming."
To appreciate the longevity of the retiring Form 222, consider that the New York Times compared the use of carbon paper to "pounding your laundry against a rock instead of using a washing machine" in an article published in 1998. That's right, carbon paper was judged old-timey 21 years ago.
But it worked. Caitlin Denney, office administrator at Poland Veterinary Centre in Ohio, observed that the form has been easy to use, needing to be filled out only once to yield three copies.
That doesn't mean users are nostalgic about the old form. "Happy to leave carbon behind. Scan and email will be much easier for us," said Dr. Amanda Rizner, a veterinarian in Maine, noting that her clinic is paperless and has a high-quality scanner.
The new system streamlines the old system, which the DEA said went like this:
"[T]he purchaser retains one copy (Copy 3) of the triplicate form and sends two copies (Copy 1 and Copy 2) to the supplier so that the order for a controlled substance can be filled. The supplier completes the form by entering the actual number of packages of the controlled substance(s) shipped and the actual date shipped. The supplier retains one copy (Copy 1) of the order form sent to him/her by the purchaser, and sends the other copy (Copy 2) of the order form to the DEA Special Agent in Charge in the area where the supplier is located. Upon receiving the controlled substance(s), the purchaser writes the number of packages of the controlled substance(s) ordered, which are actually received and the date received on its copy (Copy 3). Both the purchaser and the supplier must preserve their respective copy of the order form for two years and make it available to officials of the DEA for inspection, if requested."
The agency estimates that using the single-page form will save a total of $25.9 million a year, of which $800,000 is savings by the agency in the way of less-expensive production of forms, postage and equipment maintenance. The bulk of savings, $22.1 million by the DEA analysis, will accrue to drug purchasers using Form 222, "primarily due to efficiencies gained from having more lines per form, anticipated reduction of instances of form failure, allowing the use of a printer and general ease of use." The agency notes that purchasers will incur new copying expenses but calculates that, even so, each purchasing entity will net an average $312 in savings per year.
Suppliers who dispense Schedule I and II substances — individual or institutional practitioners such as doctors, pharmacies, hospitals and clinics that are registered to dispense a controlled substance and may distribute controlled drugs to another practitioner — stand to save $2.9 million a year, the DEA says, due to having the option to email a completed Form 222 to the DEA rather than use standard mail. Likewise, manufacturers and distributors ("non-dispensing suppliers") of controlled drugs will see $200,000 in savings, the DEA calculates, because they no longer will be required to mail copies of completed order forms to the agency.
The old Form 222 doesn't expire immediately. Users may continue using their remaining stock of carbon-paper triplicates until they run out or until Oct. 30, 2021, whichever comes first. For more details about the change, see the DEA's final rule.
This story has been changed from the original to correct the date when the carbon triplicate form is fully phased out.