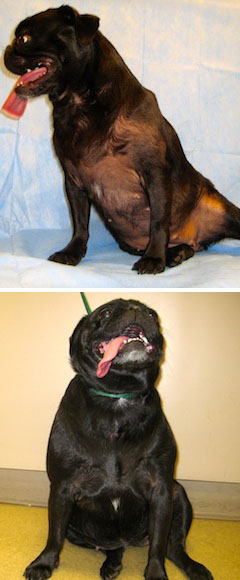
Photos by Dr. Darren Berger
A 3-year-old pug inadvertently exposed to her owner’s topical hormone therapy product developed baldness and swollen nipples (top). After the owner stopped using the hormone spray, the dog recovered, as shown in a photo taken six months later (bottom).
Three pugs from the same household, two females and one male, different ages and unrelated, shared a perplexing problem: Their fur was falling out.
The balding, known as alopecia, particularly was bad in two of the dogs. Their undersides were almost bare, and their chests and upper limbs sported thin, patchy fur. It so happened that those two pugs spent more time than the other sitting on their owner’s lap. Coincidence?
Nope. Once a group of veterinary dermatologists figured out what was going on, the fact that the two more-cuddled dogs had more severe fur loss made perfect sense. The cause of the pets’ trouble was contact with an estrogen hormone therapy that the owner sprayed on her arms.
The cases of the balding pugs are among six described in a paper published in the March/April issue of the Journal of the American Animal Hospital Association. The paper by lead author Dr. Darren Berger and fellow veterinary dermatologists is the first to document alopecia in dogs caused by exposure to human topical hormone replacement therapy (THRT).
It is only the second article in the scientific literature to document any clinical effects in pets of accidental contact with their owners’ hormone treatments.
In the lay media, the phenomenon has been reported extensively by the VIN News Service starting in 2010 and mentioned in a New York Times blog. The VIN News Service has logged anecdotal reports of more than 100 suspected cases dating to 2003.
Until now, the only report of the issue to appear in a scientific journal was published July 15, 2008, in the Journal of the American Veterinary Medical Association under the title “Theriogenology Question of the Month.” It describes a single case of secondary hormone exposure involving a female bichon frise who had bloody discharge from a swollen vulva at the age of 4 months — abnormally young for estrus.
Berger said that paper, while worthy, did not convey the potential scope of the problem. “It was one of those ‘Oh, gee whiz’-type things that’s in the front of the journal” that left a variety of aspects unanswered, he said, including defining diagnostics.
Berger said he and his co-authors, veterinarians with whom he worked at the specialty clinic Dermatology for Animals in Gilbert, Arizona, aimed to provide more information, addressing questions such as “What do we know? How are these cases coming in? What are the abnormalities?”
Berger said the phenomenon is absent from medical references. “There’s just nothing out there. If you review dermatology textbooks, even the new ones, I don’t think there’s anything mentioned in there,” he said.
The dermatology specialty hospital saw its first case in 2009. The patient was a 19-month-old castrated male Boston terrier who began losing his fur a year earlier. The dog had had a case of mange that was successfully treated with ivermectin, and he was bathed frequently with a benzoyl peroxide shampoo. But his baldness became progressively worse. After a change in diet didn’t help, he was referred to the dermatology clinic, where he was examined by Dr. Anthea Schick.
Diagnostic tests, including biopsies and blood work, suggested an underlying endocrine disturbance. The dog’s testosterone levels were normal, but he had elevated levels of other hormones, including estradiol — a form of estrogen — and progesterone.
When Schick discussed the findings with the dog’s owner, the owner mentioned that she was enrolled in a clinical trial for a hormone-replacement therapy known as the Wiley Protocol involving a compounded cream that she applied to her forearms.
Schick advised the owner to discuss alternative treatments with her physician. Three-and-a-half months after the woman stopped using the hormone cream, her pet’s coat was fully regrown.
The case served as an “aha” moment for the clinic, said Berger, crediting Schick’s diagnostic skill. “She was the first one to specifically identify what the problem was,” he said. “She was the one who brought it to our attention.” Schick is one of the paper's five co-authors.
The household of three pugs came to the clinic two years later. One of the dogs was a 3-year-old spayed female, the second was a 4½-year-old castrated male, and the third was was a 5½-year-old spayed female. They had a variety of issues in addition to fur loss. The younger female dog had an enlarged vulva, vaginal discharge and swollen nipples. The older female had an enlarged vulva. The male dog had swollen nipples and enlargement of the prepuce, or foreskin.
When the dogs’ veterinarian interviewed the owner about possible exposures common to the three dogs, the owner said that she began using Evamist, a commercial spray-on hormone preparation, four months before her youngest pug developed clinical signs.
“Interestingly, the owner noted that the two most severely affected dogs spent more time sitting on her lap than the less affected dog,” the study authors wrote.
All three dogs had elevated estradiol, and two had elevated progesterone. Testosterone levels were normal in all cases.
Once the owner stopped using the hormone product, all three dogs recovered within four months, with “complete resolution of their alopecia and symptoms of feminization,” the veterinarians reported.
The case spurred the dermatologists to submit the findings for publication. “It was the household of pugs that convinced us that we needed to get something out there,” Berger said. “It was from the standpoint that, hey, we just saw a bunch of dogs show up with a kind of unique problem confined to the household and … although it was a known entity, there was nothing out there in the reference literature.”
Within six months of the pugs’ case, as the dermatologists were in the process of writing their paper, another pair of cases surfaced. The patients were unrelated basenjis from the same household. One was a 7-year-old castrated male who’d had progressive alopecia for the past year — a condition that developed about six months after he was adopted into the household. The second was an 11-year-old female who’d been balding for 2½ years. The female basenji also had a history of Fanconi syndrome, a genetic kidney disease.
In addition, the nipples of both dogs were enlarged, and the female dog had a moderately enlarged vulva.
It turned out that the dogs’ owner, like the owner of the pugs, was using Evamist. As with most of the other dogs, the basenjis had elevated levels of estradiol and progesterone and normal levels of testosterone.
And consistent with the other cases, when the owner stopped using the hormone product, the dogs recovered — within 4½ months in the case of the male basenji, and within 5½ months in the case of the female basenji.
In order to characterize secondary hormone exposures for scientific publication, the dermatology clinic covered the cost of lab work for the pugs and basenjis, understanding that the owners, once they knew the probable cause, likely would be disinclined to pay for the biochemical details.
“You’re looking at a couple hundred dollars to run baseline hormone levels each time they’re done,” Berger said. “From the interest of science, it’s great, but from the standpoint of owners, most don’t want to pay that.”
For the purposes of diagnosing secondary hormone exposure in pets, the study authors caution that while all six dogs showed elevated baseline blood levels of estradiol and four had elevated baseline progesterone levels, a separate study published in 2010 found that estradiol levels can vary greatly in normal dogs.
“It would be advisable that in cases of suspected THRT exposure, baseline hormone levels be used to support a diagnosis and not be used as a definitive test,” the dermatologists write. “Diagnosis of hyperestrogenism, regardless of the cause, should be based on patient history, clinical signs, a lack of diagnostic test results compatible with the more common causes of canine alopecia and baseline serum sex hormone levels.”
Berger, now an assistant professor of dermatology at Iowa State University College of Veterinary Medicine, said he has not come across any cases in the university’s small animal hospital, but he hears of cases seen by colleagues. “People are recognizing it and it’s not being brought up as often as (before), but I suspect that it is still going on,” he said.
Cynthia Stuenkel, MD, a clinical professor of medicine at the University of California, San Diego, School of Medicine and a former president of the North American Menopause Society (NAMS), shares that suspicion.
Since she learned in 2010 from a VIN News Service reporter about the accidental-exposure risk, Stuenkel has worked to spread the word on the human medical side. She collaborated with VIN, an online community for the profession and parent of the VIN News Service, to develop a research poster, which she presented at the 2011 annual meeting of the Endocrine Society. Stuenkel also arranged for NAMS to post a notice online calling attention to the inadvertent exposure of pets and children to topical estrogens.
Getting the word out sufficiently is difficult in light of the therapy’s popularity in America, she said, citing new research published in the journal Menopause that estimates between 1 million and 2.5 million women aged 40 and older in the United States use compounded hormone products. That study's authors estimate that compounded products, often marketed as “bioidentical” hormones, account for somewhere between 28 percent and 68 percent of all hormone-therapy prescriptions.
“I anticipate that these problems (of accidental exposure) will continue to plague animals ... as the use of compounded bioidentical hormones is higher than we thought,” she said by email, “and in spite of NAMS’ efforts, owners seem unaware of the possibility of transfer with transdermal therapies …”
The effort to raise awareness continues bit by bit. In the Endocrine Society’s new Clinical Guideline on Treatment of Symptoms of Menopause, presented at the organization’s annual meeting earlier this month, Stuenkel said, “We indicate that gels and sprays can be transferred to persons and pets!”