Hearing screenshot

Screenshot from livestream of House Subcommittee on Economic and Consumer Policy hearing
Flanked by photos of dogs purportedly killed by Seresto flea and tick collars, U.S. Rep. Raja Krishnamoorthi (left) called on the U.S. Environmental Protection Agency to take steps to stop the sale of the collars, during a June 15 congressional subcommittee hearing. No veterinarians testified at the hearing. Many in the profession aren't seeing problems with the collar in their practices.
 Listen to this story.
Listen to this story.
It is with a sense of déjà vu that staff at animal hospitals have been fielding a flurry of calls from pet owners worried about Seresto flea and tick collars. More than a year has passed since USA Today reported that the popular product was implicated in hundreds of pet deaths.
Fresh alarm has been stoked by federal lawmakers who, at a hearing in June, called for the collars to be taken off the market. Their concern is based on what they say are an unacceptably high number of adverse-incident reports and claims that the collar is now "linked" to more than 2,500 pet deaths.
In a 23-page report, the lawmakers cited rejection by Canada of the collars in 2016 and internal emails at the U.S. Environmental Protection Agency to demonstrate that serious concern exists in the regulatory ranks.
Some veterinarians, however, continue to maintain that the evidence doesn't present a consistent or coherent picture of heightened risk for a collar that is effective at protecting pets against fleas and ticks.
Several veterinary toxicologists told the VIN News Service that whether the collar is the true cause of reported harms is unclear. Adverse-event reports are unverified and anecdotal, they say, and drawing conclusions from that raw data is imprudent.
What's more, practitioners are not seeing red flags in their day-to-day experience.
"If you poll toxicologists and the veterinarians who recommend millions of these collars … it's a nonissue," Dr. Sharon Gwaltney-Brant, a board-certified veterinary toxicologist, told VIN News. "But in today's world, any jury or congressional committee can decree to 'know' things that science cannot support."
Directors of two national animal poison centers — Pet Poison Helpline and the ASPCA Animal Poison Control Center — have reported no deaths associated with the collars.
In a recent message board conversation on the Veterinary Information Network, an online community for the profession and parent of the VIN News Service, practitioners said they don't see worrisome trends with Seresto.
"It's hard to filter through the noise," said Dr. Paul Pion, VIN president and co-founder, who has been watching the conversation. "Most veterinarians aren't seeing [problems with the collars] … but regulators believe they are picking up a signal. Someone has to figure it out."
A more complete picture could be available this fall, when the EPA, which regulates the collars and other pesticide products used on pets, completes a comprehensive reevaluation of incident reports. At that time, the agency will determine whether additional safety measures are needed, according to an EPA spokesperson.
Pet Poison Helpline's experience
Seresto cat and dog collars are designed to slowly release small amounts of the active ingredients flumethrin and imidacloprid for months at a time. The collars were brought to market in 2012 by Bayer Animal Health, which was acquired by Elanco Animal Health in 2020. More than 33 million collars have been sold, according to Elanco President and CEO Jeffrey Simmons.
Dr. Ahna Brutlag is well acquainted with the collars. She is the director of veterinary services and senior veterinary toxicologist for Pet Poison Helpline, a 24-hour animal poison control service that takes calls from pet owners and veterinarians, and provides veterinary toxicological/product safety consulting expertise to professional, scientific and nongovernmental organizations and industry, including Bayer.
"The collars themselves are not often causing significant harm," Brutlag said, referring to what she's deduced from Helpline data, as well as a review of adverse-incident fatality reports from Bayer and what's available in the public domain.
From January 2013 through March 2021, the service received 408 calls about the collars with no confounding factors (like the pet also ate a chocolate bar). Most of those calls, 86%, involved a dog chewing or swallowing the collar.
Oral exposure poses a higher risk because "the active ingredients in the collar are designed to stay on the surface level of the skin," said Brutlag, adding that veterinarians typically worry more when a collar is ingested.
Among the 408 patients, there were zero fatalities reported. In almost all of those cases — 99.5%, or 406 pets — clinical signs were not serious or life-threatening, Brutlag said: "The vast majority of cases in which clinical signs occurred were mild, transient and self-resolving." Common signs included vomiting, lethargy, diarrhea and anorexia. Clinical signs in the two cases in which the pets had serious or life-threatening illness were determined to be inconsistent with and unrelated to exposure to the active ingredients in Seresto, Brutlag said.
Brutlag's work also includes evaluating adverse-event reports, which are at the heart of the current Seresto uproar. "Just looking at the raw numbers can be deceiving," she said. Not only are the reports often medically unverified, she explained, they can be taken out of context.
For example, the most recent up-to-date count of 98,000 incidents sounds like a lot, but considering more than 33 million collars have been sold, only a small fraction are involved. "It's also helpful to know that the majority of incidents are minor in nature, such as hair loss or skin irritation at the collar site," she said.
Brutlag believes that the collars may act as a seemingly obvious explanation for signs of illness, even if they are not the cause. "I think the fact that people can see the collar on their pet, it might lead them to think that it is the problem," she said.
Dr. Tina Wismer, senior director of the ASPCA Animal Poison Control Center and a toxicology consultant for VIN, told VIN News last year that the signs reported in complaints don't fit with what you'd expect from the collar.
"Looking at these reports, these are very random things, ranging from ruptured eardrums — which I can't make fit really at all — to liver failure, to heart problems, to kidney failure," she said at the time. "The fact that the signs are very random makes me think that probably [the collar] is not involved."
A poll of VIN members in late June found that, among veterinarians with patients using Seresto collars, the majority saw no adverse reactions. Of those who did see reactions, mild skin pathology was by far the most common. One respondent reported a death associated with the collar.
Some veterinarians have suggested counterfeit collars may be behind some adverse reaction reports. Knockoffs with harmful ingredients can be hard to tell from the real thing. However, last year, the EPA told VIN News it had no information on the possible counterfeiting of Seresto collars.
The American Veterinary Medical Association has opposed calls for the immediate withdrawal of the EPA registration for Seresto collars. Registration is required to sell the product.
"AVMA's interest is in ensuring the integrity of the process for the analysis of adverse events and whether reported adverse events are causally related to a product," the organization said in a statement emailed to VIN News. "Before deciding whether or not to cancel this product's registration, there needs to be a thorough review conducted by appropriate experts."
Hearing depicts alarmed regulators
In June, the House Subcommittee on Economic and Consumer Policy released the report that makes its case against Seresto. It was based on USA Today articles; a trove of EPA documents brought to light via Freedom of Information Act (FOIA) requests by the Center for Biological Diversity, a nonprofit environmental organization; and the subcommittee's own investigations.
In its report, the subcommittee highlighted Canada's analysis of U.S. adverse-reaction reports that it used in its 2016 decision not to allow the sale of Seresto collars in that country.
Canada's Pest Management Regulatory Agency (PMRA) "closely reviewed 961 'Death and Major' pet incidents using enhanced data provided by Bayer, and found that the Seresto collar probably or possibly caused 737 — or 77% — of them," the report states. "More broadly, PMRA expressed great concern over the 'number and severity' of animal incidents linked to the Seresto collar."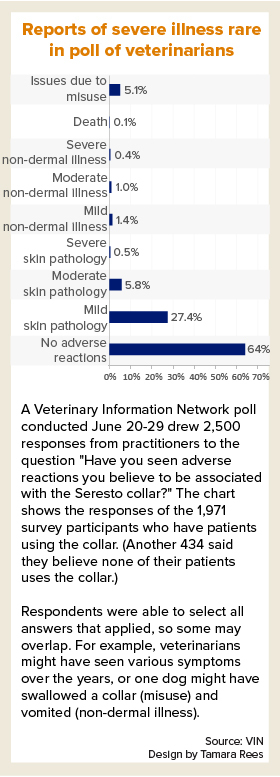
PMRA identified that among the 737 cases, nearly 80% of the animals suffered from at least one skin effect. "Most of these skin effects were serious — they covered large areas of the body, did not resolve after the collar was removed, or required medical treatment," the report states. Other frequently reported signs among the cases included lethargy, abnormal behavior, excessive grooming and vocalization, vomiting, diarrhea and anorexia.
In 2016, Canada made its causality analysis available to the EPA.
EPA independently reviewed 251 pet deaths evaluated by their Canadian counterpart and found an even stronger connection between Seresto collar use and fatalities, according to the report. PMRA had concluded that 33% — or 84 — of the deaths were probably or possibly caused by the collar. EPA concluded that 45% — or 113 — of the deaths were probably or possibly caused by the collar.
The subcommittee report also states that EPA scientists worried about the collars were directed to keep mum about those concerns, according to an internal EPA document and a subcommittee staff interview with an agency whistleblower.
After the USA Today report in 2021, an official from the California Department of Fish and Wildlife emailed EPA seeking reassurance that the collars were safe to use on endangered San Joaquin kit foxes. When an EPA official asked for the best person to answer the question, an agency scientist responded, "It depends if you want the real answer or some talking points to cover our ass for doing nothing," according to the subcommittee report.
The same scientist wrote in an email to someone else, "Looks like the sh** has hit the fan … Will be interesting to see where this goes. I hope there is a FOIA for all communications on this so that our emails are made public. We have been screaming about this for many years."
Elanco responds
Simmons, Elanco's chief, appeared at the hearing to defend the collars. "No product is without risk," Simmons told the committee in a prepared statement. "What matters is whether those risks are reasonable, in light of the benefits. Numerous studies and the incident report data for Seresto demonstrate the product does not pose an unreasonable risk."
He took issue with the title of the hearing: "Seresto Flea and Tick Collars: Examining Why A Product Linked to More than 2,500 Pet Deaths Remains on the Market."
"I must respectfully point out that the subcommittee's title for this hearing, suggesting that 2,500 pet deaths are 'linked' to Seresto, insofar as it implies some kind of causation, is simply not correct," he said.
"Elanco's pharmacovigilance team has not identified any deaths caused by Seresto's active ingredients," he continued, adding that any deaths related to the collar were caused by it becoming trapped on a structure or object, a potential issue for any collar.
Simmons reminded the subcommittee that pesticide collars are vital in providing convenient, long-term protection against serious health risks, such as tick-borne illnesses like Lyme disease. He said that the U.S. Centers for Disease Control and Prevention had supported approval of the collar to combat Rocky Mountain spotted fever.
The subcommittee report also referenced CDC support. However, it claimed CDC's desire to use the collars in a study led to a "rushed" approval process for Seresto.
EPA taking steps
A few days after the hearing, Melissa Sullivan, a spokesperson in the EPA's Office of Public Affairs, told VIN News by email, "EPA continues to take unprecedented steps to address concerns related to Seresto pet collars and other pesticide products used on pets."
In April 2021, the EPA requested from Elanco and Bayer additional information on reported adverse effects. It received the information the following month.
"This additional information was more extensive than what is routinely reported by pesticide product registrants to EPA's Incident Data System," Sullivan explained, "and included ... detailed sales data, data on annual incident rates and severity, and any incidents in other countries where the collars are sold."
The agency is reviewing the new data with help from the U.S. Food and Drug Administration Center for Veterinary Medicine (FDA CVM).
Sullivan said the EPA sought the assistance because FDA CVM regulates animal drugs, including certain drugs to control fleas and ticks, and has "extensive expertise" in animal-safety evaluation, ongoing monitoring and adverse-event reporting for animal drugs.
The EPA expects to finish the review of incident data by fall, when it will determine whether Seresto pet collars "can still be used safely according to the instructions on the label or if additional safety measures or cancellations are needed for these products," according to Sullivan.
On a parallel track, the EPA is reviewing more than 5,400 public comments it solicited in response to a petition from the Center for Biological Diversity, which requested that the agency cancel the Seresto registration. After completing its evaluation of the incident data, the EPA will respond to the petition and the public comments, Sullivan said.
In May, the EPA's Office of Inspector General "announced that it would begin evaluating whether EPA's response to reported incidents of unintended effects from pet collar pesticides was consistent" with its statutory responsibilities, Sullivan said.
Correction: The description of a VIN member poll result has been revised. Originally, the story reported that of veterinarians who saw reactions to the collar, mild skin pathology was by far the most common, at 27%. That is incorrect. Twenty-seven percent is the proportion of veterinarians reporting mild skin reactions out of all the veterinarians who said they have patients that wear Seresto collars.