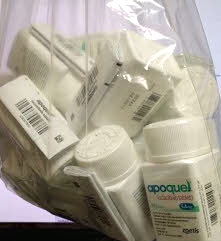
Photo courtesy of Dr. Richard Sproc
Apoquel tablets come in three strengths (3.6 mg, 5.4 mg or 16 mg) of oclacitinib maleate, a synthetic inibitor of the Janus kinase family of enzymes. Dogs take Apoquel twice daily for two weeks and once-a-day thereafter. Pictured above is one veterinarian’s recent shipment of the coveted anti-itch drug as supply woes continue to frustrate many in the profession.
A prescription drug considered by some to be a godsend to itchy dogs has soured in the mind of many veterinarians and pet owners, and not because it doesn’t work.
It's because they're struggling to get it.
Apoquel (oclacitinib tablet) has been on perpetual backorder or short supply since soon after its launch in January 2014. The drug is designed to control itching and inflammation associated with allergic dermatitis and clinical manifestations of atopic dermatitis, sometimes within 24 hours of use.
Veterinarians say communication from manufacturer Zoetis about drug availability has been subpar, at best, which is feeding the frustration.
“I have clients who have heard all about the drug from wherever they have heard it and quite frankly, some have indicated that they think we are lying when we say we do not have any because they know people who have dogs on it,’” said Dr. Lisa Ethridge of Flagstaff, Arizona. “One client went to the dermatologist and paid for an exam in order to get Apoquel because that is the only place where she could find it.”
Ethridge is one of many colleagues venting about Apoquel supply woes on the Veterinary Information Network (VIN), an online community for the profession. The complaints stem from nearly 18 months of rolling shortages, order rationing, unkept supply promises and what some believe is preferential treatment by drug maker Zoetis to stock some practices with Apoquel and not others.
“I hold great animosity towards this company and its policies,” Ethridge continued on VIN. “They have made my job, which is already very difficult, even harder. … You know what I would like? A massive media campaign from Zoetis that tells the public that their vet is not crazy or lying and that the company screwed up royally and has out and out lied to the vets and claimed that they can produce a drug that they cannot.”
Officials with Zoetis say they “share the frustration” and are working hard to resolve supply challenges. Spokeswoman Colleen White explained that the company no longer is accepting new orders for Apoquel until supply issues are resolved.
“Until supply stabilizes, we recommend that veterinarians limit their use of Apoquel to dogs already on therapy,” she said by email.
Zoetis has a policy to sell medications only through veterinary practices, yet online pharmacies sometimes obtain them through gray market channels. Owing to supply difficulties, the drug is out of stock at 1-800-PetMeds, the Internet's largest pet pharmacy.
Specifics on how and when manufacturing might catch up with demand are unclear. Zoetis officials say they've delayed plans to launch Apoquel in countries such as Canada and Australia in order to prioritize supply for existing customers.
A company statement added, “Please be assured that everyone at Zoetis is committed to stabilizing our supply so that we can make Apoquel available to all dogs that need it as soon as possible.”
That’s done little to placate veterinarians who’ve been prescribing the drug since its 2014 launch but weeks later were told it was backordered, just as clients were asking for their first refills. Demand for the drug, designed to mitigate a dog’s urge to scratch, often within 24 hours of use, quickly outpaced supplies — a major problem considering that Apoquel is rumored to take up to two years to manufacture.
Last summer, Zoetis pledged that manufacturing improvements would put an end to the supply troubles by April, prompting more veterinarians to prescribe the drug to patients. Across the country, some 13,000 veterinarians now have ordered the drug, more than double those who were able to offer it to clients and patients in 2014, White said.
The fix was short-lived.
Zoetis officials have not given a reason for the continued manufacturing problems. Along with the recent moratorium on new customer orders, allocations for current customers will be “adjusted” in June and July, White said.
In a statement, officials explained that the amount of active ingredient in the tablets hasn’t changed, but many practices are receiving less than what they typically order: “We are holding steady on the amount of Apoquel customers have ordered in two doses — 3.6 mg and 5.4 mg — to help ensure that we can supply their existing patients with Apoquel. We did have to reduce their 16 mg dose by two bottles.”
Some practitioners lament that they cannot reliably refill their clients’ prescriptions. Others have heard they aren’t on the short list to be restocked because they aren’t American Animal Hospital Association (AAHA) members.
Dr. John Daugherty is among those “beyond fed up” with Zoetis. Like Etheridge, he aired his frustration on VIN.
“When we first ordered Apoquel, we got several bottles of each size,” wrote Daugherty, owner of Poland Veterinary Centre near Youngstown, Ohio. “When the supply problem started, we were told ‘it's coming soon’ … as we all have been, for well over a year. Then they said that the amount we were ‘entitled to’ depended on how much we ordered initially, so we should be OK.
“Fast forward to about a year ago, when I decided to drop my AAHA membership for a variety of reasons. Suddenly, my Apoquel dried up. I am lucky to get one bottle of one or two sizes every two months, at best. Pissed off a lot of owners as well as all of us.”
Daugherty said he was told by a veterinary drug distributor that an AAHA membership ensured five to seven bottles a month. “In my mind, this is extortion at best,” he objected.
The VIN News Service was unable to reach AAHA, a trade group that certifies veterinary hospitals, to ask whether it has such an arrangement.
Zoetis' White said that's not how the drug maker operates. "We prioritize customers based on continuity of care for dogs," she said.
Dr. Richard Sproc of Titusville, Florida, is not an AAHA member and has experienced few supply problems compared with some of his colleagues.
He decided to try Apoquel after it launched and initially received 20-count bottles of tablets in each available strength: 3.6 mg, 5.4 mg and 16 mg.
When he tried to reorder, 3.6 mg tablets were backordered. "I had better luck getting in several bottles of 5.4 and 16 mg tablets," he said on VIN. "We managed by not starting many large-breed dogs on Apoquel and dividing tablets for smaller patients."
In June 2014, Sproc's Zoetis reps signed him up to automatically receive 100-count bottles of Apoquel every 60 days, and he received a few bottles of 5.4 mg and 16 mg tablets "fairly regularly."
Lately, his shipments have doubled. Last Friday, he posted a photo of a bag filled with Apoquel bottles on VIN as a sign that supplies soon might normalize.
“The Big Brown Truck just left. Don’t give up hope,” he said.