Dog with Cushing's
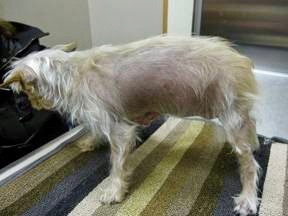
Photo by Dr. Murray Moffatt
Accurate measurements of cortisol levels are important in diagnosing and monitoring the endocrine diseases Cushing's and Addison's. One visible sign of Cushing's as it progresses is alopecia, or hair loss, as seen in the pictured dog.
Dr. Kathleen Freeman received an email in October on a topic that sent some of her team at the German pathology company Synlab into a frenzy.
The message was from the head technician at the company's veterinary laboratory in Hitchin, England, asking her to take an urgent look at some canine cortisol readings that were precipitously lower than normal.
The readings seemed to indicate that the animals were suffering from dangerously low levels of the hormone cortisol — or there had been some kind of error.
The stakes were high. Low cortisol levels point to Addison's disease, which can be fatal. On the flip side, unusually high levels of cortisol are a sign of Cushing's syndrome, also a serious condition. A deceptively low measurement could lead to an incorrect diagnosis of Addison's or a missed diagnosis of Cushing's.
The readings sent for Freeman's review were produced by the Siemens Immulite 2000 immunoassay system, a piece of diagnostic equipment used in both veterinary and human medicine for years — only there had been a recent change to the machine's set up. The Hitchin laboratory was the first at Synlab's United Kingdom operations to receive a new cortisol test kit, which Siemens was in the early stages of rolling out across Europe, ahead of distribution elsewhere, including North America.
Could it be possible, Freeman and her colleagues wondered, that the new antibody in the test kit was throwing the results out of whack? To find out, they started comparing readings from the new kit in Hitchin with readings at other labs still using the old kit.
"We were all working like crazy between the labs trying to collect the data, analyze the data and compare the data," Freeman, a senior veterinary clinical pathologist at Synlab, told the VIN News Service from her home in Seattle, where she recently relocated from Falkirk, Scotland. "There was quite a lot of panic."
A week or two earlier, on Oct. 9, Dr. Peter Graham, working from his home in Leicestershire, England, received an email with a similar message. Graham is a clinical associate professor of endocrinology and clinical pathology at the University of Nottingham School of Veterinary Medicine and Science. He also heads the Endocrine Quality Assurance (EQA) program, a voluntary program created by the European Society of Veterinary Endocrinology (ESVE) to monitor the accuracy of diagnostic tests.
The email to Graham came from someone at a different laboratory in the U.K. saying they had generated a batch of unusually low cortisol readings from the Siemens Immulite 2000. Graham responded by asking dozens of other EQA-member laboratories if they had similar issues, and he set up an online spreadsheet for data sharing. Among those who provided information to him was Freeman at Synlab.
"We were able to get up to very large numbers quickly, and the discrepancy became very apparent," Graham told VIN News.
It turned out there was indeed an issue with the new assay material in the Siemens Immulite 2000. In an initial review, the EQA found that, based on more than 400 analyses of blood serum in dogs, the average reading was 23% lower than what laboratories and veterinarians were accustomed to. The effect was even more marked in canine urine cortisol, for which the average readings were 70% lower than typical, based on runs of more than 40 samples.
Siemens, a German engineering giant, swiftly moved to rectify the discrepancy by applying mathematical adjustment factors, which it released in early November. VIN News is unaware of any animals being misdiagnosed before Siemens recognized the problem.
Questions remain over whether the adjustment factors are sufficient to offset the negative bias, particularly for urine samples. More broadly, the incident has raised wider questions about whether the veterinary diagnostic realm needs to be more tightly regulated.
What exactly went wrong?
Siemens first telegraphed the upcoming assay change in a customer bulletin issued on Sept. 29. The original antibody pool for the assay had run out and had to be replaced. Siemens said the new kit lots gave results "clinically equivalent" to the previous lots and would be rolled out to laboratories starting Oct. 5 in Europe.
Because the EQA was onto the problem by Oct. 9, Graham said, there was only a short period during which irregular results could have caused a problem for veterinary clinics. At the same time, he noted, not all laboratories are in the network, so it's possible that others did not become aware of the problem as quickly. According to Graham, 90 laboratories participate in the EQA scheme, with around 40 of those using the Siemens Immulite 2000. (The EQA scheme is run free of charge to participants and supported by sponsorship donations to the ESVE by animal drug companies Dechra Pharmaceuticals and MSD Animal Health.)
Antech Diagnostics, one of the largest veterinary diagnostic companies in the world, said it does not use the Siemens Immulite 2000. Idexx Laboratories, another large laboratory company, declined to comment.
Graham said the lower-than-usual readings stemmed, in part, from the Immulite 2000 being designed primarily with human medicine in mind. While the molecular structure of cortisol is identical in different species, he explained, there are differences among species in how cortisol is transported in the body and how related steroids react in the test. (Siemens, in its instruction guide for the Immulite 2000, cautions that cortisol results may be skewed by cross-reactivity with another steroid, 11-deoxycortisol.)
In its Sept. 29 bulletin, Siemens included a standard statement recommending that laboratories use commercially available quality control (QC) material, which can be analyzed to pick up any changes in equipment performance. However, most commercial QC is designed to detect assay malfunction involving human samples. Siemens also suggested that labs establish "an appropriate internal laboratory QC scheme," which could entail storing QC material derived from comparable animals.
Still, Graham said the fact that Siemens had sprung a new antibody testing reagent on laboratories on short notice was far from ideal. "To give us an announcement on the 29th of September that a new kit was coming out on the 5th of October for an assay that had supply issue intermittently already — which meant people were hungry for stock — is an impossible situation to put labs in," he said.
Freeman expressed disappointment, too. "Most kit manufacturers, when they have a change, they let you know far enough ahead that you can run them in parallel for a while and test them out," she said. "They didn't on this one."
A Siemens spokesperson confirmed in a statement by email that the original verification and validation testing on the cortisol kit was performed only with human samples "in accordance with the product's design and intended use." She also confirmed that the company, after implementing the new assay material, had received inquiries from veterinary labs expressing concerns.
How effectively has the problem been fixed?
In November, Siemens wrote to its Immulite 2000 customers in the veterinary realm telling them it had solved the issue by devising a "regression equation" that aligns results with the new antibody more closely to historical values. It developed a barcode that automatically integrates the regression equation, such that users need make no adjustments to the results. Starting in January, Siemens also intends to supply a "veterinary use only" test kit that is distinct from the cortisol kit used for human medicine.
Asked how many veterinary laboratories use the Immulite 2000, Siemens declined to say. The company spokesperson also would not say whether the new test kit has been released in the U.S. yet, or to specify how effective its solution is.
Graham at the EQA said the fix appears reasonably effective for blood tests but less so for urine tests, judging from an ESVE analysis. Its data indicates that when the regression equation is applied, blood results are still around 8% lower than average, which is better than the 23% negative bias seen earlier. But the urine values are still about 60% lower, compared with 70% initially. Urine tests, although not always as reliable as blood tests, can be popular as an early diagnostic tool because they are easier and cheaper to do.
"The difference that this method has had on the measurement of cortisol in urine is so massive that this new formula cannot correct that," Graham said.
To mitigate against misdiagnosis, he said, laboratories may want to consider changing their interpretive cutoff values when performing a standard urine test, known as the urine cortisol-to-creatinine ratio (UCCR) test. A cutoff value is the level of a substance, such as cortisol, that is chosen to determine whether a disease can be ruled out. "There's a little bit more freedom to make those changes in UCCR anyway because there's already quite a bit of variety of cutoff levels around the world," Graham said.
Cushing's is a relatively common condition, which, according to one study, is diagnosed in one or two dogs per 1,000 each year. Clinical signs include excessive thirst, hair loss — especially around the trunk — and a potbelly.
While a urine test may help screen for the condition, definitive diagnosis entails multiple blood analyses. The preferred diagnostic test for Cushing's is called low-dose dexamethasone suppression (LDDS), which requires an injection of the drug dexamethasone and three serum cortisol readings over the course of several hours.
Addison's is diagnosed through an adrenocorticotropic hormone (ACTH) stimulation test, in which patients are injected with the synthetic hormone Cortrosyn, with blood samples taken before and after the injection.
Dr. Sherri Wilson, an internal medicine specialist and consultant at the Veterinary Information Network, an international online community for the profession and parent of the VIN News Service, explained that the UCCR test is a broad sweep. "Yes, it's positive in almost all of our Cushing's cases but it's also positive in a lot of dogs for various other reasons besides Cushing's," she said. "So while it's a sensitive test, it isn't at all specific."
Wilson said using UCCR may be appropriate when a practitioner wants to rule out Cushing's — for example, where a dog is showing one clinical sign, such as drinking lots of water, but isn't displaying other signs, such as hair loss or a potbelly. "If you think the dog actually has Cushing's, I'd skip the UCCR and just go with the LDDS or ACTH stimulation test," she said, noting that LDDS is preferable for Cushing's.
For Addison's, Wilson said, practitioners rarely use an initial urine test, and more typically screen for the condition with a resting serum test. If cortisol levels are below a certain point, a practitioner might proceed to an ACTH stimulation test. The upshot is that accurate measurements are key to diagnosing and monitoring both conditions.
"The cortisol levels in all of these tests are how Cushing's or Addison's are diagnosed," Wilson said. "There's no question that it's a big deal if the assay changes, as the labs will have to scramble to figure out whether the normal ranges and the cutoff values for diagnosis are still valid."
A call for tighter regulation
Although Siemens acted quickly to address the problem once it was identified, the incident spotlights an area of veterinary medicine that some say is too sparsely regulated.
Dr. Stijn Niessen, a past president of the ESVE, worries that it took a voluntary program like the EQA, which he cofounded in 2012, to confirm the extent of the problem.
"I know from my colleagues in human medicine that their testing regimes aren't all that regulated either, which is sort of outrageous, because this is the basis of diagnosis," Niessen told VIN News. "Some test results can lead to life or death decisions, [and] some of them ... can lead to a lifelong application of medication that turned out not to be necessary."
Manufacturers of medical devices for animal use in the U.S. and Europe are subject to relatively little regulation compared with manufacturers of devices for human use. For instance, the U.S. Food and Drug Administration does not require manufacturers of devices for animal use to seek pre-marketing approval from the agency, nor are they required to register their businesses or list their devices, including diagnostic equipment. By contrast, in Australia, suppliers must apply for inclusion of their devices on a national register before they can be sold, and veterinary laboratories are required to carry out some quality assurance protocols.
Niessen, an endocrinology consultant at VIN and an honorary internal medicine professor at the Royal Veterinary College, said that laboratories or antibody manufacturers often change testing components or entire assays without performing a thorough validation. Before the Siemens incident, he said, external verification exercises by the EQA found an alarmingly broad variation in cortisol readings among different laboratories all using the same sample material. Recent EQA reports also indicate that some laboratories use the Siemens Immulite 2000 to test levels of the hormone insulin, even though the device is less apt at measuring animal insulin than human insulin.
The incident involving the Siemens test kit for cortisol, though, stands out for its breadth of impact. "We're used to having little esoteric things. And every few years we'll lose an assay because the manufacturer's changed how the assay works," Graham said. "The reason this had a bigger impact is because cortisol is very commonly measured and result interpretations are based on a lot of background that's already out there in print."
Niessen would like to see quality assurance protocols that validate testing procedures in a timely fashion made mandatory in Europe and elsewhere, particularly for serious conditions that are tricky to diagnose, such as endocrine diseases. "What you need is in-field validation and re-validation when you change one of the testing ingredients," he said. "And you should do that even when the ingredients don't change, really, because other things might change without you knowing it. You might have a new technician, or your machine is in a new location and therefore, the temperature is different."
On the other hand, Freeman cautions that regulation is a double-edged sword because it costs money. "The old saying in the laboratory world is that 'I can give you a high-quality test, I can give it to you fast and I can give it to you cheap. Now, which two out of the three do you want?' " she said.
Furthermore, Graham warns, placing a heavier regulatory burden on engineering companies such as Siemens could prompt them to stop offering certain tests to the veterinary community.
As a relatively straightforward solution, he suggests that laboratories store animal QC material derived from surplus serum and use it to check that a new testing method works correctly for the species in question. Doing so, he said, wouldn't cost much because laboratories could, with appropriate consents, freeze serum left over from other activities, rather than disposing of it.
Veterinarians also should be mindful of clinical signs, he advised, and not rely strictly on test results that may not always be accurate. "I think this situation is reminding people that we're not treating numbers — we're treating animals," he said.
Freeman concurs: "You have to look at what the animal's doing. Is he more active? Is he eating better? Is he happier, brighter clinically? And certainly we can use additional laboratory tests in conjunction with that, but for many of the endocrine diseases, you really need to monitor the clinical signs to know."