Hill's
Screenshot from Facebook.com
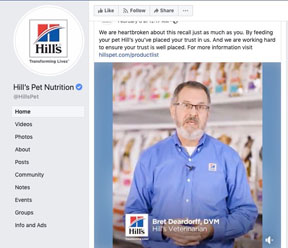
In a video posted to Hill's Facebook page, Dr. Brett Deardorff, senior manager of veterinary affairs, says the company is "heartbroken" about the recall.
Click here for a larger view
A growing recall prompted by potentially dangerous levels of vitamin D in Hill's Pet Nutrition canned dog foods appears to have marred the company's reputation with veterinarians and pet owners, leading the company to post a public apology.
Dr. Brett Deardorff, senior manager of veterinary affairs, said Hill's has "isolated and identified" how more than 30 varieties of canned dog food contained toxic levels of vitamin D. In video posted Feb. 6 to Hill's Facebook page, he states that the problem is being corrected.
Hill's is doing additional testing with respect to vitamin D.
"We are heartbroken about this recall," Deardorff said. "Hill’s people are working, all hands on deck, with veterinarians and pet parents to listen to any and all concerns. ... We have tripled the number of people on our phone and extended our call-center hours. We are assessing every pet parent inquiry thoroughly."
"Human error" by a vitamin supplier led to the recall on Jan. 31 of 25 varieties of Hill’s canned dog food, Hill’s officials said. More lots were withdrawn Feb. 8 because they did not meet the company’s formula specifications but not because they posed a health risk, officials said.
Hill’s manufacturers and sells dog food under Hill’s Prescription Diet and Hill’s Science Diet labels. The earliest manufacture date of impacted canned dog food was August 2018, with shipping starting Sept. 1, 2018.
"We're examining this very, very thoroughly," Dr. Jolle Kirpensteijn, chief professional veterinary officer at Hill’s U.S., said in an interview with the VIN News Service. "There was a supplier error in the manufacturing of vitamin premix."
He added, "We realize that this is not good for pet patients, pet parents or veterinarians."
The Hill's incident follows a recall late last year of dry dog foods that were found to have excessive, potentially toxic levels of vitamin D. More than a dozen brands of dog food were recalled between Nov. 2 and Dec. 20. Hill's officials say there's no connection between that spate of recalls and theirs.
"Our supplier is a well-known supplier based in the U.S.," Kirpensteijn said. "We’re really not aware if there’s any relationship with other brands recalled in December."
Hill's officials declined to share the supplier's identity.
Greg Aldrich, a research associate professor and coordinator of the Pet Food Program at Kansas State University, made a seemingly prophetic statement to the VIN News Service following the recalls in November and December. "The perception is that boutique pet food makers have more problems than the large companies. But the bigs have just as many issues," he said.
High levels of vitamin D can sicken animals and in rare instances, cause death. Overdoses can lead to a range of signs in dogs, from drooling, constipation and/or vomiting, to seizing. Increased urination and thirst also are commonly reported. Vitamin D poisoning can increase levels of calcium in the blood, a condition known as hypercalcemia. Too much calcium in the blood can induce bone loss and abnormally high serum calcium levels, which could result in kidney stones and the calcification of organs like the heart and kidneys if left untreated.
Kirpensteijn declined to put a number on deaths or adverse reactions tied to the recall, saying the company is still reviewing reports from pet owners and veterinarians.
A number of pet owners have posted on social media and other online forums about the illnesses and deaths they believed were caused by Hill’s diets. One is Lori Kroeger, a licensed veterinary technician in Rochester, Washington, who said her two pugs got sick and died after eating Hill’s Prescription Diet W/D Canine, one of the varieties later recalled by the company.
Resources for veterinarians
"I am heartbroken, furious and disgusted," she wrote in a joint message board discussion of the Veterinary Support Personnel Network and the Veterinary Information Network, online communities for the profession. "I have 15-plus years of experience in the veterinary field and because of that, I'm probably a little better equipped to handle and understand what has happened, but I can't imagine what the average consumer of your product is going through."
Anne Norris, a health communications specialist at FDA, says the agency received just one adverse event report from Hill’s, dated Jan. 30, which prompted the recall. "To our knowledge, that’s the first case," she said.
Since then, Norris said, the FDA has received more than a dozen adverse event reports from pet owners and veterinarians. "The cases are still coming in," she said this week, adding, "But some people don't know that they can report directly to our agency."
Meanwhile, veterinarians are fielding questions they say Hill's has not prepared them for. On VIN, veterinarians lament that they’ve recommended Hill’s for their use of nutritionists, research, ingredient sourcing and quality control, only to have their faith in the company broken.
Dr. Deborah Adelsohn of Morris Plains, New Jersey, aired her frustration in a Feb. 2 post to colleagues on VIN.
"As a company with a long, excellent reputation, I would have thought Hill’s would have done more to try and save it," she wrote. "They BLEW IT! … The phone was ringing off the hook yesterday. More specific action on what to look for, how to test and what to do with recalled foods should have been made available. Our rep eventually returned our call and only said, 'Put recalled stuff off to the side and I'll get back to you.' "
Adelsohn added: "I have been a Hills fan for over 25 years. This one is going to take a long time to get my trust back."
Dr. Stephen Steep of Eastpointe, Michigan, stated much of the same: "There are pet food recalls weekly, but this is a diet we send out our door on a daily basis. ... Just wish Hills had taken the time to email or fax us directly with this information."
Asked why Hill’s didn’t reach out to veterinarians first, Kirpensteijn replied that the company had to prioritize its response to the recall. "The main concern — this may be a little bit harsh — is the pet," he explained. "The FDA is very clear that you have to reach out to the consumer, which we did exactly at the same time we reached out of the vets. We used a lot of our internet [capabilities] to do that.
"… It’s frustrating for everyone, but we are working around the clock to give as much information as soon as possible."
Hill’s recall spurs class actions
At least three class actions were filed against Hill's Pet Nutrition this week on behalf of pet owners. The lawsuits are open for other pet owners impacted by the recall to join. Attorneys for Hill’s have not responded to litigation.
Bone v. Hill’s
Plaintiffs in Florida, North Carolina and New York are leading a suit filed Feb. 11 in U.S. District Court for the Eastern District of New York.
Kelly Bone, Christina Sawyer and Janine Buckley allege that Hill’s sold specialty dog foods containing "toxic and often fatal levels of vitamin D" and that the company knew about it months prior to its recall on Jan. 31 of canned Prescription Diet and Science Diet products. The suit alleges that dogs owned by each of the plaintiffs died as a result of consuming the tainted Hill's products.
"Not only has Hill’s sold contaminated food, but it has dragged its feet in issuing a recall and including all contaminated food within the scope of the recall," the lawsuit alleges.
The Chicago-based firm Cafferty, Clobes, Meriwether & Sprengel LLP represents the plaintiffs.
Navarette v. Hill’s
John Navarrete of California is suing Hill’s on behalf of himself and other prospective plaintiffs in a suit filed Feb. 12 in U.S. District Court for the Northern District of California.
Navarette alleges that his German Shepherd, Goliath, became ill after eating cans of Hill’s Prescription Diet that later were recalled. In December, Goliath was taken to an emergency veterinary hospital and referred to the University of California, Davis, veterinary medical teaching hospital, where he recovered after treatment and a recommended change in diet. "As a result," the lawsuit says, "Navarrete incurred substantial veterinary bills."
The lawsuit accuses Hill’s of falsely advertising its pet foods as nutritious and safe for consumption and alleges that the company knew certain cans of dog food might contain excessive vitamin D amounts prior to the recall. It seeks monetary damages and injunctive relief to prevent Hill's from selling pet food with dangerous levels of vitamin D, and requests a jury trial.
The San Francisco-based firm Schubert, Jonckheer & Kolbe is handling the case.
Russell v. Hill’s
Michael and Jodi Russell of Florida are lead plaintiffs in a suit filed Feb. 11 in U.S. District Court for the Northern District of Florida. The lawsuit alleges that Hill’s dog food deemed "defective" due to excess vitamin D "poisoned" the Russell’s dog Stella, who was euthanized on Jan. 26.
"Plaintiffs first became aware of the recall after Stella’s death when Mrs. Russell saw a newscast about the recall and realized the connection between Stella ingesting the product, her physical symptoms, and her ultimate kidney failure and death," the lawsuit reads. "Mr. Russell spoke with the family vet on Feb. 8, 2019, and was advised that, in the veterinarian’s opinion, ingestion of the product was most likely the cause of Stella’s kidney failure. The veterinarian pointed out that the blood work performed before Stella ingested the product showed normal renal function, but after ingesting the product over many days Stella went into renal failure."
The lawsuit seeks at least $5 million in aggregate damages from Hill’s. Court documents say more than 100 pet owners are anticipated to join the case, which is being handled by two law firms: Levin, Papantonio, Thomas, Mitchell, Rafferty & Proctor in Florida and Reese LLP in New York.