 Federal regulators continue to receive reports of suspected food-related cases in pets of dilated cardiomyopathy (DCM), a potentially fatal heart disease.
Federal regulators continue to receive reports of suspected food-related cases in pets of dilated cardiomyopathy (DCM), a potentially fatal heart disease.
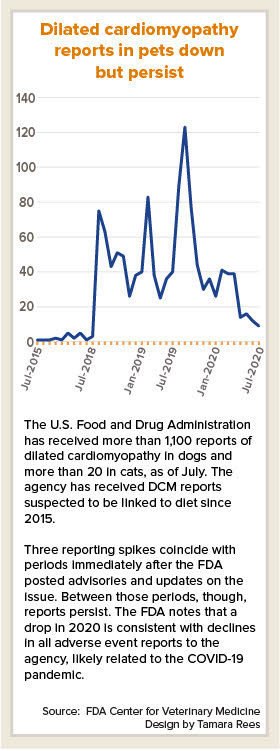
In an update provided at a scientific forum last month, officials from the U.S. Food and Drug Administration Center for Veterinary Medicine said that as of July 20, the agency had received more than 1,100 reports of DCM in dogs, and more than 20 reports in cats. The majority of the reports were made since 2018.
In DCM, the heart is enlarged and weak, unable to pump blood efficiently. Dogs with DCM might tire easily, cough and have shortness of breath. More severe clinical signs include fainting or collapse. The disease can cause sudden death.
In the past, DCM in dogs was found only in particular breeds known to have a genetic predisposition, including Doberman pinschers, boxers, Great Danes, Irish wolfhounds and cocker spaniels. The FDA became aware that something was amiss when veterinary cardiologists notified the agency that they were seeing DCM in a variety of other breeds. A common thread that emerged in the atypical cases was a grain-free diet.
The FDA first alerted the public about the issue in July 2018.
Exactly what it is about a grain-free diet that might cause or contribute to the illness is unclear. Meanwhile, pet foods labeled as grain-free remain popular and readily available.
In remarks at the Sept. 29 conference, named the Scientific Forum Exploring Causes of Dilated Cardiomyopathy in Dogs, the director of the FDA Center for Veterinary Medicine, Dr. Steven Solomon, called the issue a "scientific puzzle."
"I want to be clear," Solomon said. "We at CVM currently do not view this as a regulatory issue. We have not requested any recalls. We have not taken any compliance or enforcement activity. This is a matter of science ..."
In addition to FDA scientists, Solomon said, cardiologists and nutritionists from the veterinary community, members of the Pet Food Institute trade group's nutrition subcommittee, and individual companies have contributed to the investigation.
Similarly, the conference, which was hosted by Kansas State University and conducted virtually because of the COVID-19 pandemic, drew presentations from researchers in academia and industry, as well as government.
Among the presentations was a look by the FDA at fully and partially recovered cases of DCM in dogs.
Of the 1,100-plus cases reported to the agency, the FDA is closely following a subset of 161 cases. That subset is further divided into two groups.
The first group is comprised of 121 cases that were reported to the agency between January 2018 and April 2019. Of those, 23 patients (19%) have fully recovered, and 84 patients (69%) have partially recovered. Seven died. The rest had no improvement or their condition was unknown.
Tellingly, all of the dogs that fully recovered received a change in diet. Most also were treated with taurine, an amino acid; and pimobendan, a cardiac drug.
In most cases, recovery took seven to 13 months after their initial diagnosis.
In the second group are 40 dogs whose cases were reported to the FDA between November 2019 and July 2020. All have had a diet change, and 75% have fully or partially recovered.
A slide in the presentation notes that "the data are fluid and changing ... and more dogs will likely recover."
Update: On Nov. 3, the FDA issued a statement by Dr. Steve Solomon, director of the agency's Center for Veterinary Medicine, about the Kansas State University scientific forum, along with a Q&A about the FDA's work on potential causes of non-hereditary DCM in dogs. This is not "an investigative update," the agency noted, but "an inflection point that provides FDA with an opportunity to clarify and emphasize some key points about non-hereditary DCM."