Ban on powdered gloves coming Jan. 18
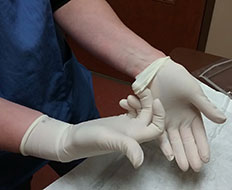

Photo courtesy of Valley Animal Hospital
Powdered medical gloves, such as this supply at a veterinary hospital in New Jersey, must be thrown away to comply with a federal ban that takes effect this month.
Powdered medical gloves are going the way of powdered wigs.
A once ubiquitous staple of doctors, powdered gloves are being thrown out of exam and operating rooms by the U.S. Food and Drug Administration as of Jan. 18. The reason: The powder poses a variety of risks to wearers, patients and even bystanders.
The dangers include severe airway inflammation from inhaling the powder; wound inflammation and post-surgical adhesions from contact with the powder; and respiratory allergic reactions from breathing powder that carries proteins from natural rubber latex gloves. The most common type of powder used in gloves is cornstarch, according to the FDA.
The coming ban is absolute — there’s no grace period for using up existing supplies. “[T]he risks of illness or injury to individuals who are currently exposed to these devices is [as] equally unreasonable and substantial as it would be for future individuals that might be exposed to powdered gloves,” the FDA stated in a March 22, 2016, Federal Register notice proposing the ban. The ban was made final on Dec. 19.
Although glove use in veterinary medicine is not explicitly mentioned in the FDA rule, the prohibition applies in the veterinary sphere, too, an agency spokeswoman confirmed.
“The ban applies to powdered surgeon’s gloves, powdered patient examination gloves, and absorbable powder for lubricating a surgeon’s glove that are already in commercial distribution and for these devices that are already sold to the ultimate user, such as small medical practices and hospitals. As such, it applies to ... gloves that are in use at veterinary practices,” the spokeswoman, Deborah Kotz, said by email.
Asked how the ban will be enforced, Kotz replied: “The FDA can take various enforcement actions, if necessary, to remove banned devices from the market, including seizure of the product, civil money penalties or criminal prosecution.”
She declined to say what criminal charges could be brought, or the potential size of fines.
The FDA recommends unused inventories of gloves be disposed of like any community solid waste, which usually is by burial in a landfill or by incineration.
Dr. Bruce Henderson, hospital director of Valley Animal Hospital in Clifton, New Jersey, estimates that his practice has $150 worth of powdered gloves in stock. “I’m just going to pitch them all in the garbage and buy new ones,” he said in a message-board discussion on the Veterinary Information Network, an online community for the profession.
Henderson said he wouldn’t want to risk creating a situation in which employees claim harm from the use of banned gloves. Moreover, he’s already largely made the transition to powder-free gloves and prefers them.
“My associate requested non-powdered gloves when she started working here a few years ago, so we switched over. I like the non-powdered way better!!” he wrote on VIN.
Henderson explained by email that he likes not getting powder all over himself when he removes the gloves.
Some veterinarians are less enthused about switching.
VIN News Service photo
Dr. Karen Vanderloo, a practitioner in Wisconsin, has been dissatisfied so far with powder-less gloves. She hopes her clinic will find other styles that work better. “I’m sure we’ll all get used to the new gloves eventually,” she said.
Dr. Karen Vanderloo, a veterinarian at Oregon Veterinary Clinic near Madison, Wisconsin, is unimpressed with the performance of non-powdered gloves.
“Anticipating the change, we got a shipment of the powder-free gloves about six to eight weeks ago, and the general consensus was not favorable,” she told the VIN News Service by email. “They’re more difficult to put on, especially immediately after scrub prep before surgery, and because of the rolled cuff, are harder to put on in sterile fashion — the rolled edge keeps folding/rolling on itself.”
Other practitioners cite the difficulty of donning powder-less gloves with sweaty hands. That’s one advantage of powdered gloves, the FDA noted. “The benefits of powdered gloves appear to only include greater ease of donning and doffing, decreased tackiness and a degree of added comfort …” the agency stated in its notice of the final rule.
These benefits, the FDA concluded, “are nominal when compared to the risks posed by these devices.”
Long history of problems
The use of lubricant powders in surgical gloves dates to the late 19th century. At the time, the powders consisted of the spores of Lycopodium, an evergreen herb also known as club moss.
“By the 1930s, Lycopodium powder was recognized to cause wound granulomas and adhesion formation and was replaced by talcum powder (chemically, hydrous magnesium silicate) … In the 1940s, talcum powder (talc) was also recognized to be a cause of postoperative adhesions and granuloma formation. In 1947, modified cornstarch powder was introduced ...” according to the FDA.
Despite changes in powder type, problems persisted. In 1997, FDA issued a Medical Glove Powder Report that described the risks of glove powder and the state of the medical-glove market. Because no good alternatives to powdered gloves existed at the time, the agency opted not to ban them: “The report concluded that banning powdered gloves in 1997 would cause a market shortage of medical gloves, which could result in inferior glove products and increased costs to the U.S. health care system …”
Public pressure caused the FDA to revisit the issue some years later. Between 2008 and 2011, the agency received three petitions asking it to ban the use of cornstarch powder on latex and synthetic surgical and examining gloves.
One of the petitions accompanied a report published by the American Journal of Emergency Medicine in 2009 discussing the dangers of cornstarch powder on medical gloves. The authors stated that Germany banned surgical glove cornstarch powder in 1997, and that the United Kingdom’s purchasing and supply agency stopped purchasing gloves lubricated with cornstarch in 2000.
In 2011, the FDA put out a call for public comments on the risks and benefits of powdered gloves.
The agency also considered issuing a formal warning about the risks of gloves, but, as explained in the rule finalizing the ban, concluded that warning labels would be inadequate:
“[P]atients often do not know the type of gloves being worn by the health-care professional treating them, but are still exposed to the potential dangers. Similarly, glove powder’s ability to aerosolize and carry NRL (natural rubber latex) proteins exposes individuals to harm via inhalation or surface contact. Thus, some of the risks posed by glove powder can impact persons completely unaware or unassociated with its employment and without the opportunity to consider the devices’ labeling.”
Perhaps just as compellingly, the agency now believes that the market easily can handle the switch. “Our searches … revealed that the market is saturated with alternatives to powdered gloves, resulting in downward pressure on the prices of non-powdered gloves. In addition, the share of powdered medical gloves sales has been declining since at least 2000, while total sales of all disposable medical gloves have increased.”
Glove manufacturers largely have supported phasing out powder. In an interview published by the magazine Infection Control Today in late 2015, representatives of several manufacturers said unequivocally that the health concerns are valid. They also said alternative gloves are abundantly available. A representative of Halyard Health (formerly Kimberly-Clark Health Care) said her company sells only non-powdered exam gloves. Medline Industries' representative said his company offers 20 different powder-free options with synthetic polymer coatings inside the gloves to make donning and double-gloving easier.
Henry Schein, a leading distributor of medical, dental and veterinary supplies, states on its website that it carries “a wide selection of powder-free latex medical exam gloves manufactured by reputable companies,” and names seven makers plus its own private-label brand.
The FDA cites statistics suggesting that the timing of the ban should be no trouble for the vast majority of practitioners: “[R]ecent projections of annual gloves sales indicate that at least 93 percent of medical providers have switched to non-powdered gloves.”
The FDA notes that while manufacturers will be prohibited as of Jan. 18 from importing powdered gloves, they may export powdered gloves to countries where they are lawful. The agency does not address the ethics of exporting products that it has judged to present an unacceptable health risk.
VIN News Service staff writers Christy Corp-Minamiji and Phyllis DeGioia contributed to this report.