GettyImages-123459829
NNehring/E+ via Getty Images
The FDA has drafted alternative approaches for evaluating heartworm prevention in dogs.
The U.S. Food and Drug Administration intends to update its standards for evaluating the efficacy of new canine heartworm preventatives in response to drug-resistant strains of Dirofilaria immitis, the parasitic worm that causes the disease.
The prospective changes could draw new broad-spectrum parasiticides to the U.S. market.
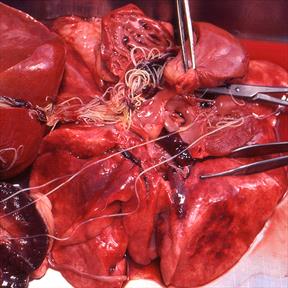
Spread by the bite of infected mosquitoes, heartworms can inhabit the arteries of a dog's lungs and the heart, causing clinical disease that commonly manifests as coughing and lethargy but sometimes can be fatal. While dogs are primary hosts, cats, ferrets, otters and other species have been infected.
But in some parts of the country, the parasite has developed resistance to macrocyclic lactones, a class of parasiticides and insecticides that includes ivermectin, selamectin, milbemycin oxime and moxidectin — active ingredients in major brands of injectable, tablet and topical preventatives. The FDA has re-imagined its approach for evaluating new canine heartworm preventatives since 2018, owing to questions about product effectiveness and the limitations of studies conducted to support product approval.
In November, the FDA released a draft of the proposed new vetting process titled "Effectiveness of anthelmintics: Specific recommendations for products proposed for the prevention of heartworm disease in dogs." Anthelmintics are antiparasitic drugs used to expel worms.
The public is invited to weigh in on the draft document until May 1, when the comment period ends. Feedback can be submitted via the Federal eRulemaking Portal, or in writing to Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm 1061, Rockville, MD 20852.
The FDA's current approach to ensuring that canine heartworm prevention products are 100% effective involves requiring sponsors to conduct two separate laboratory dose confirmation studies using research dogs and recent isolates of D. immitis from two U.S. regions, and one multisite field effectiveness study.
The draft guidance is similar in terms of the number and types of recommended studies but includes specific recommendations to better study product effectiveness under actual conditions involving client-owned dogs, and to select at least two types of heartworm isolates circulating in highly endemic areas, such as the southeastern United States and Mississippi Delta Region. "The field study recommendations are intended to provide effectiveness data from a broader population to better understand the actual effectiveness in the target population, because the wildtype heartworm population cannot be replicated in a lab study," the FDA said.
The new approach also lowers the efficacy mandate to 99.5% — a change that could attract makers of products sold outside of the United States.
"There are some really good molecules that are in broad-spectrum products in other countries, that we can't get here because they're not 100% effective," said Dr. Michael Dryden, a professor of veterinary parasitology in the Department of Diagnostic Medicine and Pathobiology at Kansas State University. "It would be great for that to change, because it could benefit a lot of animals."
Dr. J. Scott Weese, an infectious disease veterinarian at the University of Guelph Ontario Veterinary College and VIN consultant, applauds the FDA's efforts. "It's good to see clear directions about what needs to be done for new approvals, and it seems pretty straightforward. There's definitely some resistant heartworm around, mainly in some pockets in the southeastern U.S. It doesn't seem too widespread, but it's present."
The Companion Animal Parasite Council estimates that more than 100,000 dogs in U.S. are diagnosed with heartworm disease annually.