Ignatios C. Liapis, DVM, CES d' Ophtalmologie ENVT
During the last years, clinical electrophysiology has considerably improved, allowing the evaluation of the retina and visual pathways in many species. Electroretinography (ERG) is a electrophysiological technique which measures the retinal action potentials in response to light stimulation. In small animal practice, electroretinography is more useful than other diagnostic techniques for the assessment of retinal function. Even though ERG is performed by specialist veterinary ophthalmologists, general practitioners must be informed about this diagnostic procedure.
MORPHOLOGIC/PHYSIOLOGIC CHARACTERISTICS OF THE RETINA
The retina has 10 layers. The retinal pigment epithelium (RPE) is the outermost layer strongly adherent to the choroid. The more important functions of RPE are the transport of nutrients from the choroid to the outer retinal layers and the phagocytosis of outer segments of photoreceptors as they are continually shed. The nine remaining layers of the retina form the neuroretina. These nine layers are considered from outside inward:
 The photoreceptor layer, which contains the outer and the inner parts of the photoreceptors where the light absorbing pigmented molecules, are located.
The photoreceptor layer, which contains the outer and the inner parts of the photoreceptors where the light absorbing pigmented molecules, are located.
 The outer limiting membrane
The outer limiting membrane
 The outer nuclear layer
The outer nuclear layer
 The outer plexiform layer
The outer plexiform layer
 The inner nuclear layer
The inner nuclear layer
 The inner plexiform layer
The inner plexiform layer
 The ganglion cell layer
The ganglion cell layer
 The nerve fiber layer and
The nerve fiber layer and
 The inner limiting membrane. Muller cells are glial cells, which are vertically oriented in the neuroretina providing support and nutrition for the retina.
The inner limiting membrane. Muller cells are glial cells, which are vertically oriented in the neuroretina providing support and nutrition for the retina.
There are two types of photoreceptors, the cones, and the rods. The density of cones is greatest in the area centralis and decreases toward the periphery of the retina. Cones function at photopic levels of illumination therefore they are useful for vision during daylight. Cones can rapidly response to repetitious stimulations, and provide sharp visual acuity and color sensitivity. On the other hand, cones are less sensitive to low levels of light stimuli, so that color vision is ineffective in very dim light. Rods are very sensitive to light; thus, they function more effectively in scotopic illumination but they become saturated at photopic levels of illumination and they are inactivated in daylight. The maximal absorption by rod photopigment (rhodopsin) occurs at a wavelength of about 508nm for the dog and 501nm for the cat. On the other hand, there exist two types of cones in the retina of small animals: those which contain a photopigment with maximum absorptive sensitivity in the violet (dog: 430nm, cat: 450nm), and those with maximum absorptive sensitivity in the yellow-green (dog: 555nm, cat: 560nm). Because the responses of cones and rods must be considered separately to evaluate retinal function, many of these differences of the physiologic characteristics between cones and rods are useful in the record of ERG.
Phototransduction is the physiological mechanism by which the light is transformed into electric current. In a dark environment, the photoreceptors are depolarized because of a constant influx of cations (Na+), which continually leak from the extracellular space into the outer photoreceptor segment through light sensitive channels, and pumped at the same time from the inner photoreceptor segment to the extracellular space. The activation of photopigment by photons produces a cascade of biochemical reactions leading to closure of the ion channels. The channel closure hyperpolarizes the photoreceptor. Because the photoreceptors synapse with bipolar and horizontal cells, any change of their electrical status produces a series of events that involve the other retinal cells (bipolar, horizontal, amacrines, interplaxiforms and Muller cells). ERG records the sum of these electrical changes.
MORPHOLOGY OF NORMAL ERG
There exist two types of ERG, the flash ERG and the pattern ERG, according the utilized light stimulator. Flash ERG evaluates the function of outer retina and is performed by using flashlight units that uniformly stimulate the retina. Pattern ERG, which is rarely used in veterinary practice, reflects the functioning of the inner retina by using checkerboard light stimulus.
Three primary waves of the typical canine flash ERG can be recognized (Figure 1):
 The a-wave, which is the first negative peak, refers to the hyperpolarization of photoreceptors.
The a-wave, which is the first negative peak, refers to the hyperpolarization of photoreceptors.
 The b-wave is the first positive peak, which follows the a-wave, and is principally generated by Muller cells.
The b-wave is the first positive peak, which follows the a-wave, and is principally generated by Muller cells.
 The c-wave is a late positive potential after the b-wave. The c-wave refers to the function of RPE; however it is unusual and is not examined in most cases.
The c-wave is a late positive potential after the b-wave. The c-wave refers to the function of RPE; however it is unusual and is not examined in most cases.
The implicit time and the amplitude of the a- and b-waves are used for interpretation of ERG. Implicit time is the time from the light flash (0 msec) to the a-wave trough and b-wave peak respectively. The a-wave amplitude is measured from the prestimulus baseline to the a-wave trough whereas the b-wave amplitude is measured from the a-wave trough to the peak of b-wave.
| Figure 1. | 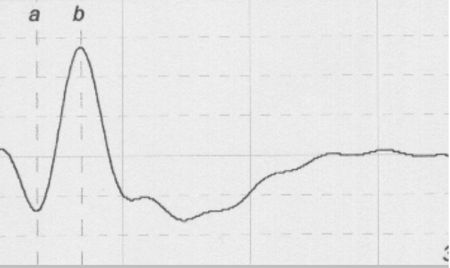
A typical canine ERG |
|
| |
FACTORS THAT INFLUENCE THE QUALITY OF THE ERG
A number of factors can cause variations in a flash ERG:
 Factors related to the conditions of the procedure: the parameters of the stimulus, the retinal adaptation to the ambient light, the background luminance during the procedure, the type of recording electrodes, the electric properties and influences of the stimulating and recording systems.
Factors related to the conditions of the procedure: the parameters of the stimulus, the retinal adaptation to the ambient light, the background luminance during the procedure, the type of recording electrodes, the electric properties and influences of the stimulating and recording systems.
 Factors related to the patient: the species, breed and age of the animal, the oxygenation of the patient and its globe, eyelids or muscle movements during the procedure, the pupil size, the depth and type of anesthesia, the transparence of the refracting tissues of the eye.
Factors related to the patient: the species, breed and age of the animal, the oxygenation of the patient and its globe, eyelids or muscle movements during the procedure, the pupil size, the depth and type of anesthesia, the transparence of the refracting tissues of the eye.
It is obvious that small animal ERGs vary according to all these factors and comparison of normal values would be difficult. Therefore, each veterinary ERG laboratory obtains their own normal values.
TECHNIQUE OF ERG
ERG involves advanced techniques and different recording equipments are in use. Whatever modern ERG unit is computerized and contains the stimulator, the electrodes, the amplifier and the signal averager and printer.
The stimulating system used by the author consists of two white unilateral xenon flashlights, which provide a luminous surface of 2cm diameter. To attenuate or change the color of stimulating light, neutral density filters or/and color can be placed in front of light stimulators.
Because ERG reflects the difference between the potential of the active and reference electrodes, these two types of recording electrodes are in use. Many types of active electrodes are available. Among the corneal contact lens electrodes, the needle electrodes and the hook-like sclero-conjunctival electrodes the author uses the scleroconjunctival hook-like electrodes, which are clipped at the bulbar conjunctiva, just posterior to the corneal-bulbar conjunctival junction, centered at the dorsal quadrant. Subcutaneous silver wires are recommended as reference electrodes that are placed at the ear tragus. A similar subcutaneous ground electrode is placed at the back between scapulas.
The recording signal must be amplified because retinal potentials are very small. Furthermore by using bandpass filters, amplifiers attenuate all frequencies below and above the low and high settings respectively.
The following protocol is followed in our practice for realization of a routine ERG procedure.
During the adaptation time, full dilatation of the pupil occurs by applying local tropicamide 1% several times. After 2 hours photopic adaptation patients are anesthetized, using medetomidine (0.05 mg/kg) and ketamine (10 mgr/kg). Then, the patients are intubated and placed in sternal recumbency. Using a vacuum beat-filled pack the head is positioned in a secure place inside a plastic frame. After clipping the conjunctiva, the sclero-conjunctival electrodes are immobilized on the plastic frame so that the upper eyelid is kept open, the globe is stabilized and the retina well exposed to the stimulating light. The corneas are protected with nonirritating methylcellulose solution. The reference and ground electrodes are correctly positioned and the two light sources are installed approximately 1 to 2 cm from the corneal surface to produce equal illumination of the entire retina in both eyes. ERG routine protocol is performed as in table bellow.
ERG INDICATIONS
ERG is indicated when retinal disease is suspected. ERG also can help to identify the cause of blindness. Furthermore, ERG is used to evaluate the progression of retinal disorders.
In small animal practice, the majority of ERGs are performed on candidates for cataract surgery. ERG was shown to be an essential preoperative procedure, especially in breeds that are predisposed to hereditary progressive retinal atrophy (PRA). Miniature and Toy Poodles and Cockers Spaniel often suffer from cataract and PRA. In these cases surgical removal of opaque lens cannot improve vision. Cataractous lens cannot restrain the retinal electric response; thus, reduced amplitudes or/and prolonged latencies in animals suffering from cataract, indicate the presence of retinal disease (Figure 2).
| Figure 2. | 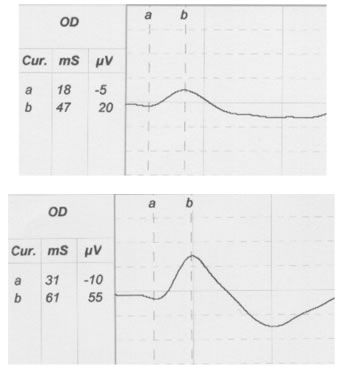
A scotopic ERG after 20min dark adaptation of a dog with early stage RPA (top) in comparison with an ERG of a normal dog (bottom). |
|
| |
ERG is useful for the early diagnosis of central or generalized PRA. Depending on the animal breed, clinical onset of PRA range from 6 weeks to 5 years. ERG helps to confirm PRA in early stages (months or years) before the appearance of the ophthalmoscopic signs so that the affected animals are excluded from reproduction. Indications of PRA include decreased b-wave amplitude, decreased flicker-fusion frequency, and changed implicit times.
ERG is also useful for the differential diagnosis between the sudden acquired retinal degeneration (SARD) and optic neuritis. Both of these pathological conditions are characterized by the onset of sudden blindness. Electrodiagnostically SARD is characterized by extinguished ERGs, whereas in patients with optic neuritis the ERG is normal.
ERG is an irreplaceable procedure for the evaluation of hemeralopia (day-blindness). In these cases flicker ERG reveals the absence of cone function, whereas scotopic ERG normally demonstrates the integrity of rods.
In glaucoma, the ERG is normal during the early stages of the disease, because the ganglion cells are damaged first. In the end stages of glaucoma, when retinal damage occurs due to chronically high intraocular pressure, abnormalities concerning the amplitude of the b-wave and the implicit time of the a-wave can be detected.
In many cases of retinal detachments (RD), only the b-wave may be altered. In other cases, the scotopic ERG waveforms are diminished, during the initial stage of the disease, while the photopic waveforms are present.
|
Test No |
Light Stimulus |
Background Illumination |
Adaptation Time |
Investigated Photoreceptors |
|
1 |
Single white flash |
photopic |
|
cones |
|
2 |
10 white 1Hz flashes |
photopic |
|
cones |
|
3 |
10 white 30 Hz flicker flashes |
photopic |
|
cones |
|
Placing the attenuation and blue filters in front of light stimulators. Turn off the light. |
|
4 |
10 blue 1Hz flashes |
scotopic |
to |
rods |
|
5 |
10 blue 1Hz flashes |
scotopic |
to+5min |
rods |
|
6 |
10 blue 1Hz flashes |
scotopic |
to+10min |
rods |
|
7 |
10 blue 1Hz flashes |
scotopic |
to+15min |
rods |
|
8 |
10 blue 1Hz flashes |
scotopic |
to+20min |
rods |
|
Removal of all filters from the front of light stimulators. |
|
9 |
Single white flash |
scotopic |
to+22min |
cones + rods |
References
1. Komaromy AM, Smith PJ, Brook DE Electroretinography in dogs and cats. Part I. Retinal morphology and physiology. Compendium of Continuing Education 1998,20:343-350.
2. Komaromy AM, Smith PJ, Brook DE Electroretinography in dogs and cats. Part II. Technique , Interpretation and Indications. Compendium of Continuing Education 1998,20:355-366.
3. Narfstrom K, Bjorn E, Serge G.R, Bernhard M.S, Christine L.P, Ron O. Guidelines for clinical electroretinography in dog. Documenta Ophthalmologica 105:83-92; 2002.
4. Sims MH. Electrodiagnostic evaluation of vision. In: Veterinary Ophthalmology, 8th ed, ed.Gelatt KN. Lippincott/Lea&Febiger, Philadelphia, 1999; 483-507.
5. Electrophysiologie Visuelle Sensorielle Appliquée à la Pratique Vétérinaire: Cours de Formation Lyon Novembre 2003 : S.V.E.R.O.V (Société Française d'Etudes et de Recherches en Ophtalmologie Vétérinaire).